B. burgdorferi ist ungewöhnlich in seiner Resistenz gegen Antibiotika-Behandlung (Preac Mursic et al. 1989) aus im wesentlichen zwei Gründen:
Es gibt grundsätzlich zwei Nischen-Typen, die vom Wirt bereitgestellte und die von B. burgdorferi geschaffene Nische.
B. burgdorferi kann als L-Form im Blutkreislauf und Liquor auch dem Immunsystem widerstehen. Über den Blutkreislauf gelangt sie in die unter 1. genannten physikalischen Nischen, in denen sie sich in die normale spirochetale Form zurückverwandeln kann, weil der Wirt dort keinen ausreichenden Zugriff auf sie hat (Mattman 1993).
Brorson und Brorson und Preac Mursic und Mitarbeiter haben in vitro nachgewiesen, daß B. burgdorferi (im kürzesten Fall innerhalb von Minuten bis Stunden, Brorson und Brorson) zwischen spirochetaler (d.h. normaler) und L-Form hin- und herwechseln kann.
| PROCESSES |
AUTHORS |
YEAR |
| Only live Bb invaded cultured endothelial cells. | Comstock, Thomas (Winston - Salem) | 1991 |
| Intracellular localization of Borrelia burgdorferi within human endothelial cells. 200 ... 5000 Bb per HUVEC -> intracellular localization of 10 ... 25% of Bb in 24 h. |
Ma, Sturrock, Weis (Salt Lake City) | 1991 |
| Invasion of human skin fibroblasts by Bb. Bb eliminated from the cell surface by ceftriaxone (1 mg/mL = 10-20 x MBC) for 5 days, residual live Bb in perinuclear region. |
Klempner, Noring, Rogers, Georgilis, Peacocke (Boston) | 1992/3 |
| Persistence of Bb in human ligamentous tissue. | Häupl, Hahn, Rittig, Krause et al. (Erlangen/Nürnberg) | 1993 |
| First isolation of Bb from an iris biopsy 4 patients IgG+/IgM- , 2 patients IgG-/IgM-. |
Preac-Mursic, Pfister, Spiegel (München) | 1993 |
| The fate of Bb in mouse macrophages: destruction, survival, recovery. reculturing of Bb from macrophages. |
Montgomery, Nathanson, Malawista (Yale, New Haven) | 1993 |
| Localization of Borrelia burgdorferi in murine Lyme borreliosis by electron microscopy. Spirochetes were generally not in or near areas of inflammation. 3 locations in hearts:
|
Pachner, Basta, Delaney, Hulinska (Natl. Inst. Public Health, Prague, Czech Republic) | 1995 |
| Ultrastruct. demonstr. of Bb antigens in synovial fluid and membrane in chronic LD. Perivascular areas, deep synovial stroma among collagen bundles, in vacuoles of fibroblasts in SM, and in cytophagosomes of mononuclear cells in SF cell samples. |
Nanagara, Duray, Schumacher Jr (KhonKaen Univ., Thailand) | 1996 |
NOTATION
Bb = Borrelia burgdorferi.
(HUV)EC = (human umbellical vein) endothelial cells.
LD = Lyme disease.
MBC = minimum bactericidal concentration.
SF = synovial fluid.
SM = synovial membrane.
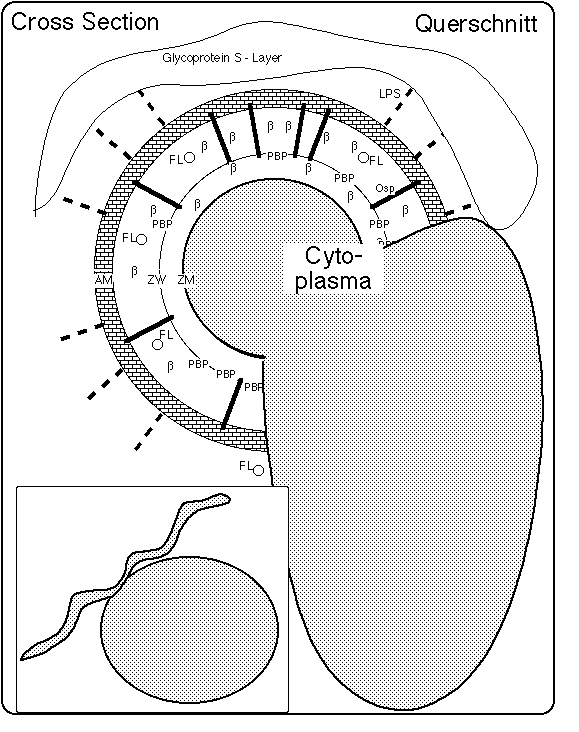
Fig. 1: Schema einer Spirochete mit Zyste: Draufsicht (unten links) und Querschnitt (nach Preac Mursic et al 1996 und Brorson, Brorson 1998). Die Oberflächen außerhalb der Zytoplasmamembran, also die Zellwand (ZW = cell wall) und die äußeren Membran (AM = outer membrane), werden durch bakterieneigene Lysozyme (auflösende Enzyme) beim Wachstum aufgelöst. Wenn durch Verwendung von Penicillinen/Cephalosporinen oder die Wirkung des Immunsystems das Gleichgewicht zwischen bakterieller Auflösung und Wiederaufbau gestört wird, entstehen zellwand-defizitäre Formen (L-Formen, auch Spheroplaste oder Zysten genannt), bei denen die Zytoplasma-Membran (ZM = cytoplasma membrane) und Flagellen von außen sichtbar werden.
Das Verhältnis von Zysten- und Spirocheten-Volumen variiert in weitesten Grenzen, von kleinen knospenähnlichen Zysten an im wesentlichen intakten Spirocheten (Preac Mursic et al 1996) bis hin zu großen Gebilden mit überhaupt fehlenden oder winzigen Spirocheten-Ansätzen (Brorson, Brorson 1998, Burgdorfer 1999).
Die Zysten können sich von den Spirocheten lösen. Sie werden dann "Blebs" genannt.
Die Spirochäte Borrelia burgdorferi hat (Seite 110 in: Atlas R)
Original article: Feder HM Jr, Johnson BJ, O'Connell S, Shapiro ED, Steere AC, Wormser GP; Ad Hoc International Lyme Disease Group, A critical appraisal of "chronic Lyme disease". N Engl J Med. 2007 Oct 4;357(14):1422-30
Epidemiol Mikrobiol Imunol. 2001 Feb;50(1):10-6.
In 18 patients with Lyme borreliosis the authors proved the persistence of Borrelia burgdorferi sensu lato by detection of the causal agent by immune electron microscopy or of its DNA by PCR in plasma or cerebrospinal fluid after an interval of 4-68 months.
PMID: 11233667 [PubMed - indexed for MEDLINE]
14th International Scientific Conference on Lyme Disease & Other Tick-Borne Disorders, June 6, 2001
Antimicrob Agents Chemother 1996 Jun;40(6):1552-4
Unite des Rickettsies, Faculte de Medecine, Centre National de la Recherche Scientifique, Marseille, France.
Despite appropriate antibiotic treatment, Lyme disease patients may have relapses or may develop chronic manifestations. The intracellular location of Borrelia burgdorferi suggests that antibiotics that penetrate cells will have greater efficiency. Doxycycline or erythromycin was more effective than penicillin or ceftriaxone in killing B. burgdorferi when the organism was grown in the presence of eucaryotic cells.
PMID: 8726038, UI: 96338386
APMIS 1998 Dec;106(12):1131-41
Department of Microbiology, Vestfold Sentralsykehus, Tonsberg, Norway.
Mobile Borrelia burgdorferi were transferred to distilled water (10(6) per ml). The cultures were observed by dark field microscopy (DFM), interference contrast microscopy (ICM) and transmission electron microscopy (TEM). 95% of the spirochetes were converted to cysts after 1 min, and after 4 h no normal mobile borreliae were observed. When transferred to growth medium (BSK-H), the cysts became smaller and more irregular, and were filled with organic substances. After 1 day, 1-5 thin structures sprouted from the cysts. They continued to grow in both length and thickness until they attained a normal spirochetal structure. Finally, these new-born spirochetes detached from the cysts, by which time their mobility had become normal. The present method for producing large amounts of cystic forms of B. burgdorferi is well suited for further studies of this unique microbe.
PMID: 10052721, UI: 99160086
Infection 1998 May-Jun;26(3):144-50
Dept. of Microbiology, Vestfold Sentralsykehus, Tonsberg.
The purpose of this study was to examine the structural alterations of Borrelia burgdorferi when exposed to spinal fluid. Normal, mobile spirochetes were inoculated into spinal fluid, and the spirochetes were converted to cysts (spheroplast L-forms) after 1-24 h. When these cystic forms were transferred to a rich BSK-H medium, the cysts were converted back to normal, mobile spirochetes after incubation for 9 to 17 days. The cultures were examined by dark field microscopy (DFM), interference contrast microscopy (ICM) and transmission electron microscopy (TEM). When neuroborreliosis is suspected, it is necessary to realize that B. burgdorferi can be present in a cystic form, and these cysts have to be recognized by microscopy. This study may also explain why cultivation of spinal fluid often is negative with respect to B. burgdorferi.
PMID: 9646104, UI: 98310072
Infection 1997 Jul-Aug;25(4):240-6
Dept. of Microbiology, Ulleval University Hospital, Oslo, Norway.
The purpose of this study was to evaluate the behaviour of Borrelia burgdorferi under controlled conditions. The occurrence of cystic forms of Borrelia burgdorferi in vitro was noted, and these cysts were able to be transformed to normal, mobile spirochetes. B. burgdorferi was cultivated in a commercial culture medium without serum. The spirochetes multiplied only slowly in this medium, and transformation to encysted forms was observed after 1 week. When these cysts were transferred to the same culture medium with rabbit serum, the encysted forms developed into regular, mobile spirochetes after 6 weeks, and their regeneration time was normal. Examination of these cysts in the transmission electron microscope revealed transverse fission inside the cysts. It is probable that similar phenomena may occur in vivo under conditions unfavourable for spirochetes. These observations may help to explain why diagnosis and treatment of B. burgdorferi infections in humans can be difficult.
PMID: 9266264, UI: 97411286
Microb Pathog 1991 Feb;10(2):137-48
Department of Microbiology and Immunology, Wake Forest University Medical Center, Winston-Salem, North Carolina 27103.
Borrelia burgdorferi can adhere to cultured endothelial cells and penetrate through cell monolayers by passing through intercellular tight junctions and through the host cell cytoplasm. Borrelia burgdorferi strains which were isolated from different sources and areas of the U.S. all demonstrated similar invasive capabilities. Bacterial penetration from the apical to the basal surface of the monolayer was 20 times more efficient than from the basal to the apical surface. Borreliae which were non-viable as a result of either heat treatment or ultraviolet (UV) irradiation showed reduced association with the endothelial cell monolayer and loss of invasive capabilities. Borreliae were able to invade when protein synthesis was inhibited with streptomycin or chloramphenicol. When assays were conducted at 4 degrees C, bacterial penetration of the monolayer was completely inhibited. Treatment of borreliae with proteases affecting outer surface proteins greatly reduced cell association and bacterial invasion.
PMID: 1890951, UI: 91367119
The American Journal of Psychiatry Special Article 1994;151(11):1571-1583.
The microbiology of B. burgdorferi sheds light on why Lyme disease is an illness that at times can be relapsing and remitting and that can be refractory to normal immune surveillance and standard antibiotic regimens.
Much of the genetic material in B. burgdorferi is contained in plasmids (76), resulting in the possibility of significant variability. This includes
Recent animal research (77) has found that the spirochete may undergo genetic alteration once it is sequestered in the CNS, thus resulting in a new strain of spirochete that is different from the infecting peripheral spirochete. The remarkable strain variation of B. burgdorferi may account for the differences between the presentation of Lyme disease in Europe and in the United States (78, 80). For example,
During growth, Bb appears to shed membranous material blebs) from its surface. These blebs coat the spirochete and have been found free in the CSF, serum, and urine (21, 82, 83).
B. burgdorferi has been shown to be capable of persisting in human hosts despite extensive antibiotic treatment (17, 85 - 88). Because in vitro studies demonstrate that B. burgdorferi
(e.g.
Several features are known to contribute to an organism's resistance to standard lengths of antibiotic treatment. These features include
B. burgdorferi appears to possess all of these characteristics.
20. Luft BJ, Steinman CR, Neimark HC, Muralidhar B, Rush T, Finkel MF, Kunkel M, Dattwyler RJ: Invasion of the CNS by Bb in acute disseminated infection. JAMA 1992; 267:1364-1367.
21. Coyle PK, Deng Z, Schutzer SF, Belman AL, Benach J, Krupp LB, Luft B: Detection of Bb antigens in cerebrospinal fluid. Neurology 1993, 43: 1093-1097.
22. Garcia-Monco JC, Villar BF, Alen JC, Benach JL: Borrelia burgdorferi in the central nervous system: experimental and clinical evidence for early invasion. J Infect Dis 1990; 161:1187-1193.
23. Loggian EL, Kaplan RF, Steere AC: Chronic neurologic manifestation of Lyme disease. N Engl J Med 1990; 323:1483-1444.
75. Garcia-Monco JC, Fernandez-Villar B, Benach JL: Adherence of the Lyme disease spirochete to glial cells and cells of glial origin. J Infect Dis 1989; 160: 497-506.
76. Barbour AG, Garon CF: Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science 1987; 237: 409-411.
77. Pachner AR, Itano A: Borrelia burgdorferi infection of the brain: characterization of the organism and response to antibiotics and immune sera in the mouse model. Neurology 1990; 40: 1535-1540.
78. Hanrahan JP, Benach JL, Coleman JL, Bosler EM, Morse DL, Cameron DJ, Edelman R, Kaslow RA: J Infect Dis 1984; 150: 489-596.
79. Steere AC, Taylor E, Wilson ML, Levine JL, Spielman A: Longitudinal assessmentr of the clinical and epidemiological features of Lyme disease in a defined population. J Infect Dis 1986; 154: 295-300.
80. Schwan TG, Burgdorfer W, Garon CF: Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun 1988; 56: 1831-1836.
81. Coyle PK: Antigen detection and cerebrospinal fluid studies, in: "Lyme Disease", Coyle PK (ed.), Philadelphia, Mosby Year Book, 1992.
82. Garon CF, Dorward DW, Corwin MD: Structural features of Bb - the Lyme disease spirochete silver staining for nucleic acids, Scanning Microsc. Suppl 1989, 3: 109-115.
83. Dorward DW, Schwan TG, Garon CF: Immune capture and detection of Bb antigens in urine, blood, or tissues from infected ticks, mice, dogs, and humans, J Clin Microbiol 1991; 29: 1162-1170.
84. Whitmire WM, Garon CF: Specific and nonspecific responses of murine B cells to membran blebs of Borrelia burgdorferi. Infect Immun 1993; 61: 1460-1467.
85. Preac-Mursic V, Weber K, Pfister HW, Wilske B, Gross B, Baumann A, Prokop J: Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection 1989; 17: 355-359.
86. Haupl T, Hahn G, Rittig M, Krause A, Schoerner C, Schonherr U, Kalden JR, Burmester GR: Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum 1993;, 36: 1621-1626.
87. Hassler D, Riedel K, Zorn J, Preac-Mursic V: Pulsed high-dose cefotaxime therapy in refractory Lyme borreliosis (letter). Lancet 1991; 338: 193.
88. Liegner KB, Shapiro JR, Ramsay D, Halperin AJ, Hogrefe W, Kong L: Recurrent erythema migrans despite extended antibiotic treatment with monocycline in a patient with persisting Borrelia burgdorferi infection. J Am Acad Dermatol 1993; 28: 312-314.
89. Georgilis K, Peacocke M, Klempner MS: Fibroblasts protect the Lyme disease spirochete, Borrelia burgdorferi, from ceftriaxone in vitro. J Infect Dis 1992: 166: 440-444.
90. Montgomery RR, Nathanson MH, Malawista SE: The fate of Borrelia burgdorferi in mouse macrophages: destruction, survival, recovery. J Immunol 1993; 150: 909-915.
91. Ma Y, Sturrock A, Weis JJ: Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect Immun 1991; 59: 671-678.
92. Mahmoud AA: The challenge of intracellular pathogens (editorial) N Engl J Med 1992; 326: 761-762.
The following reference, cited by name rather than by numbers, have been added by J. Gruber.
P. Brouqui, S. Badiaga, D. Raoult: Eucaryotic Cells Protect Borrelia burgdorferi from the Action of Penicillin and Ceftriaxone but Not from the Action of Doxycycline and Erythromycin: NOTES.Antimicrobial Agents and Chemotherapy 1996:1552‹1554.
References
17. Liegner KB: Lyme disease: the sensible pursuit of answers. J Clin Microbiol 1993; 31: 1961-1963.
Neurology 1993 Jun;43(6):1093-1098
Department of Neurology, SUNY at Stony Brook 11794.
We examined CSF for Borrelia burgdorferi antigens using antigen-capture ELISA and Western (immuno) blot. Antigen-capture ELISA was positive in 38 of 77 (49%) CSF samples obtained from neurologic patients with presumed B burgdorferi infection, compared with one of 34 (3%) CSF samples obtained from other neurologic disease controls who came from a region endemic for Lyme disease. Western immunoblot was positive for B burgdorferi antigens in 12 of 22 (55%) CSF samples from the B burgdorferi infected groups, compared with none of 11 CSF samples from the control group. CSF antigen detection should prove helpful in evaluating patients for suspected neurologic Lyme disease.
PMID: 8170548, UI: 94224329
J Infect Dis 1992 Aug;166(2):440-4
Department of Medicine, New England Medical Center, Boston, Massachusetts.
The Lyme disease spirochete, Borrelia burgdorferi, can be recovered long after initial infection, even from antibiotic-treated patients, indicating that it resists eradication by host defense mechanisms and antibiotics. Since B. burgdorferi first infects skin, the possible protective effect of skin fibroblasts from an antibiotic commonly used to treat Lyme disease, ceftriaxone, was examined. Human foreskin fibroblasts protected B. burgdorferi from the lethal action of a 2-day exposure to ceftriaxone at 1 microgram/mL, 10-20 x MBC. In the absence of fibroblasts, organisms did not survive. Spirochetes were not protected from ceftriaxone by glutaraldehyde-fixed fibroblasts or fibroblast lysate, suggesting that a living cell was required. The ability of the organism to survive in the presence of fibroblasts was not related to its infectivity. Fibroblasts protected B. burgdorferi for at least 14 days of exposure to ceftriaxone. Mouse keratinocytes, HEp-2 cells, and Vero cells but not Caco-2 cells showed the same protective effect. Thus, several eukaryotic cell types provide the Lyme disease spirochete with a protective environment contributing to its long-term survival.
PMID: 1634816, UI: 92340959
Cooperation Coyle PK, Gruber J, Hulinska D, Liegner KB, Mattie H, Draft 1999.
"In general, I.V. therapy is given until there is a clear positive response, then treatment is changed to IM or po until free of signs of active infection for 4 to 8 weeks. "
To support his approach, I have
Fig. 2: Symptom log. Horizontal axis: symptom id, vertical axis: day after onset of opticus neuritis. Each occurrence of a symptom is marked by a dot entered in the column above the name of the symptom at a vertical location given by the day of occurrence. Thin dots: during these days the corresponding symptoms appeared only in the evening, in a mild form or barely noticeable. First symptoms were
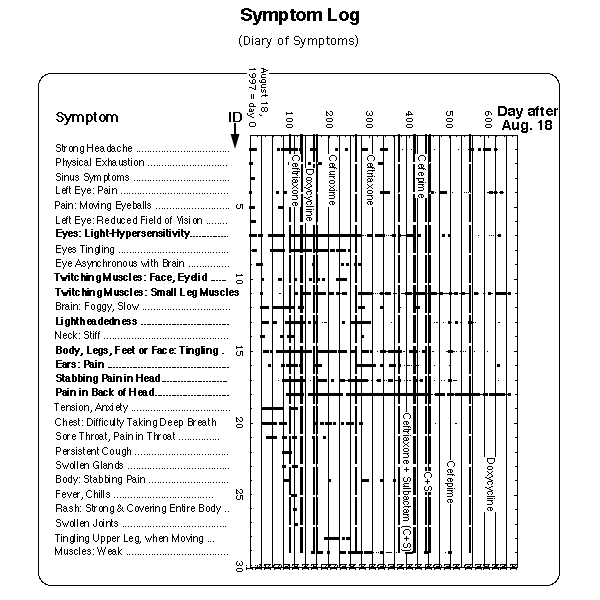
(all not entered into Fig. 2).
M = menses (data between day 170 and 220 missing). Lines with 4 weeks distance between each other have been drawn to visualize the reference flare cycle after J.J. Burrascano.
The model reflects the preference of many systems in nature for oscillations as a response to an external input (P. Ball, "The Self-Made Tapestry: Pattern Formation in Nature", Oxford University Press, Oxford, New York, Tokyo, 1999).
The following figure describes the processes incorporated in the model. The control loop depicted with heavy lines (on the left) represents the ability of the immune system to enter self-organized feedback control oscillations the period of which is determined by parameters intrinsic to the immune system. The immune system can also oscillate with a period given by the menstrual cycle (loop drawn in medium heavy lines on the right).
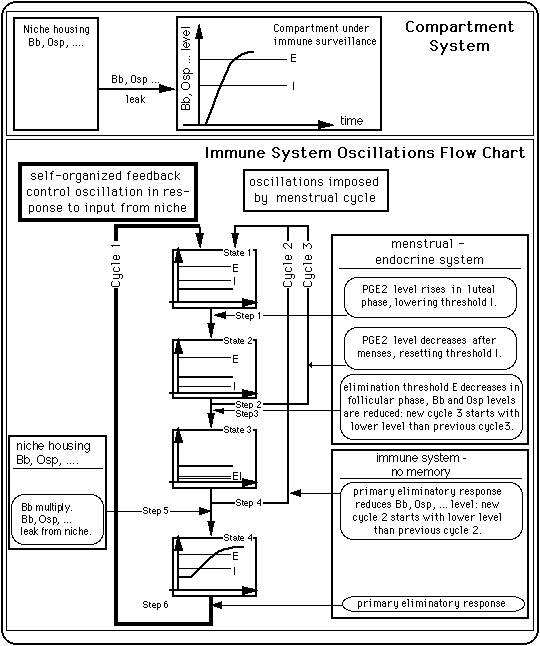
Fig. 3: A simple compartment system and an immune system control scheme that produces oscillations between an inflamed state and a symptom free state.
Mechanisms at work
It is possible that presence of cycles 2 and 3 is an indication of a low pathogen level in the niche.
Infect Immun 1998 May;66(5):2143-2153
MedImmune, Inc., Gaithersburg, Maryland 208781; Department of Biochemistry & Biophysics, Center for Extracellular Matrix Biology, Albert B. Alkek Institute of Biosciences and Technology, Texas A&M University, Houston, Texas 770302; and Microscopy Branch, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, Montana 598403
Borrelia burgdorferi, the spirochete that causes Lyme disease, binds decorin, a collagen-associated extracellular matrix proteoglycan found in the skin (the site of entry for the spirochete) and in many other tissues. Two borrelial adhesins that recognize this proteoglycan, decorin binding proteins A and B (DbpA and DbpB, respectively), have recently been identified. Infection of mice by low-dose B. burgdorferi challenge elicited antibodies against DbpA and DbpB that were sustained at high levels, suggesting that these antigens are expressed in vivo. Scanning immunoelectron microscopy showed that DbpA was surface accessible on intact borreliae. Passive administration of DbpA antiserum protected mice from infection following challenge with heterologous B. burgdorferi sensu stricto isolates, even when serum administration was delayed for up to 4 days after challenge. DbpA is the first antigen target identified that is capable of mediating immune resolution of early, localized B. burgdorferi infections. DbpA immunization also protected mice from B. burgdorferi challenge; DbpB immunization was much less effective. DbpA antiserum inhibited in vitro growth of many B. burgdorferi sensu lato isolates of diverse geographic, phylogenetic, and clinical origins. In combination, these findings support a role for DbpA in the immunoprophylaxis of Lyme disease and suggest that DbpA vaccines have the potential to eliminate early-stage B. burgdorferi infections.
Although much progress has been made in the characterization of the organism, spirochetal factors responsible for infectivity, immune evasion, and disease pathogenesis remain largely obscure. The most-studied B. burgdorferi membrane protein is outer surface protein A (OspA), a lipoprotein antigen expressed by borreliae in resting ticks and the most abundant protein expressed in vitro by most B. burgdorferi sensu lato isolates.
Arthritis Rheum 1993 Nov;36(11):1621-6
Department of Medicine III, University of Erlangen-Nuremberg, Germany.
OBJECTIVE. To document the persistence of Borrelia burgdorferi in ligamentous tissue samples obtained from a woman with chronic Lyme borreliosis.
METHODS. Spirochetes were isolated from samples of ligamentous tissue, and the spirochetes were characterized antigenetically and by molecular biology techniques. The ligamentous tissue was examined by electron microscopy. Humoral and cellular immune responses were analyzed.
RESULTS. Choroiditis was the first recognized manifestation of Lyme disease in this patient. Despite antibiotic therapy, there was progression to a chronic stage, with multisystem manifestations. The initially significant immune system activation was followed by a loss of the specific humoral immune response and a decrease in the cellular immune response to B burgdorferi over the course of the disease. "Trigger finger" developed, and a portion of the flexor retinaculum obtained at surgery was cultured. Viable spirochetes were identified. Ultramorphologically, the spirochetes were situated between collagen fibers and along fibroblasts, some of which were deeply invaginated by these organisms. The cultured bacteria were identified as B burgdorferi by reactions with specific immune sera and monoclonal antibodies, and by polymerase chain reaction amplification and Southern blot hybridization techniques.
CONCLUSION. To our knowledge, this is the first report of the isolation of B burgdorferi from ligamentous tissue. This suggests that tendon tissues serve as a specific site of spirochete residence in human hosts.
J Infect Dis 1993 May;167(5):1074-81
The ability of Borrelia burgdorferi to attach to and invade human fibroblasts was investigated by scanning electron and confocal microscopy. By scanning electron microscopy, B. burgdorferi were tightly adherent to fibroblast monolayers after 24-48 h but were eliminated from the cell surface by treatment with ceftriaxone (1 microgram/mL) for 5 days. Despite the absence of visible spirochetes on the cell surface after antibiotic treatment, viable B. burgdorferi were isolated from lysates of the fibroblast monolayers. B. burgdorferi were observed in the perinuclear region within human fibroblasts by laser scanning confocal microscopy. Intracellular spirochetes specifically labeled with monoclonal anti-flagellin antibody were also identified by fluorescent laser scanning confocal microscopy. These observations suggest that B. burgdorferi can adhere to, penetrate, and invade human fibroblasts in organisms that remain viable.
PMID: 8486939, UI: 93253286
Eur Neurol 1995;35(2):113-117
Department of Medicine, Albert Einstein College of Medicine, New York, N.Y., USA.
We report an unusual patient with evidence of Borrelia burgdorferi infection who experienced repeated neurologic relapses despite aggressive antibiotic therapy. Each course of therapy was associated with a Jarisch-Herxheimer-like reaction. Although the patient never had detectable free antibodies to B. burgdorferi in serum or spinal fluid, the CSF was positive on multiple occasions for complexed anti-B. burgdorferi antibodies, B. burgdorferi nucleic acids and free antigen.
Comments:
PMID: 7796837, UI: 95317331
after:
L-Form = Zellwand-Mangel-Form (cell wall deficient form, CWD form auch L-Form genannt, zu Ehren des Lister-Instituts). Borrelia burgdorferi in dieser Form haben eine unvollständige äußere Oberfläche und Zellwand (AM, ZW). Damit können auch Strukturen fehlen, die an diese Teile gebunden sind, z.B. die Flagellen, die Lipopolysaccharide (Seite 18 in Mattman 1993) und die Proteine der äußeren Oberfläche (Outer Surface Proteins, Osp's, siehe auch Sadziene et al. 1991, Sadziene et al. 1995).
Es ist im wesentlichen noch im Dunkeln, welche Bestandteile und Eigenschaften der Bb für die Entzündungen, Infektivität, Umgehung des Immunsystems und Krankheitsentstehung und -Entwicklung verantwortlich sind.
Journal of Clinical Microbiology Aug 1993:961-963
8 Barnard Road, Armonk, N.Y. 10504 , USALYME BORRELIOSE - Die vernünftige Suche nach Antworten
Kenneth B. Liegner, M.D., P.C.
Übersetzung von Eva Schwarz
Krankheit ist sehr alt und nichts hat sich an ihr geändert. John Martin Charcot, De l´Expectation en Medecine
Wir sind es, die sich ändern, während wir lernen zu erkennen,
was vorher nicht wahrnehmbar war.
1989 veröffentlichten Preac-Mursic et al. einen bedeutenden Artikel
(30), in dem sie die Anzüchtung von lebenden Borrelia burgdorferi
bei Patienten dokumentierten, die durch die bei ihnen durchgeführte
Therapie als geheilt galten. Dazu gehörte ein Patient, der zehn
Tage lang intravenös mit Ceftriaxon behandelt worden war und von
dessen Liquor der Organismus angezüchtet werden konnte (30). Dieser
Bericht stieß auf ungläubige Skepsis von einigen Seiten. Es wurde
die Vermutung geäußert, daß die Kulturen kontaminiert waren, oder
daß der Bericht auf andere Weise falsch gewesen sei. Seitdem sind
jedoch eine Reihe von Berichten erschienen, die das Überleben
von B. burgdorferi trotz aggressiver antibiotischer Behandlung
auch bei Anwendung der wirksamsten intravenösen Anbitiotika bestätigen
(12, 22). Diese anscheinend anomalen Beobachtungen, die die Unzulänglichkeit
des bestehenden Lyme-Paradigmas (d.h. unserer Sicht der Wirklichkeit, J. Gruber) enthüllen, sind noch immer schwer von der medizinischen Fachwelt zu akzeptieren und deuten auf eine Revolution unseres Bildes
dieser Krankheit hin (15). Solch eine Veränderung wird notwendig sein, um den biologischen Realitäten von B. burgdorferi Infektionen
wirksam begegnen zu können (1).
Die neuere Forschung beginnt Klarheit darüber zu schaffen, wie
es möglich sein kann, daß eine bakterielle Infektion sich trotz
stärkster Antibiotika ihrer Eliminierung widersetzt.
Leider reagieren einige Patienten nicht ausreichend auf Oraltherapie, insbesondere Patienten mit ernsthafter Beteiligung des Zentralen Nervensystems. Bei diesen Patienten ist möglicherweise eine längere intravenöse Behandlung erforderlich.
Abgesehen von der Vorsorge, die natürlich an erster Stelle steht, müssen Methoden zur sicheren Heilung bereits erkrankter Patienten entwickelt werden.
1a.Coburn, J., J. Leong, and J. Erban. Unpublished data.
2. Cohen, J. 1993. Naked DNA points the way to vaccines. Science 259:1691-1692.
3. Coyle, P. K., A. L. Belman, and B. Krupp. 1992. B. burgdorferi-specific
immune complexes in cerebrospinal fluid, abstr. 167, p. A29. Program
Abstr. 5th Conf. Lyme Borreliosis. 1992.
4. Coyle, P.K.. S. E. Schutzer, A.L. Belman, L.B. Krupp, and Z.Dheng. 1992. Cerebrospinal fluid immunologic parameters in neurologic Lyme disease, p. 31-43. In S. E. Schutzer (ed), Lyme disease molecular and immunologic approaches. Current communications in cell and molecular biology . Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
5. Dorward, D., E. D. Huhuenel, G. Davis, and C. F. Garen.. 1992. Extracellular Borrelia burgdorferi proteins interact with non-borrelia-directed IgM antibodies. abstr. 219, p. A38. Program Abstr. 5th Int. Conf. Lyme Borreliosis, 1992.
6. Dorward, D. W., T. G. Schwan, and C. F. Garon. 1991. Immune capture and detection of B. burgoferi antigens in urine, blood, or tissues from infected ticks, mice, dogs, and humans. J. Clin. Microbiol. 29: 1162-1170.
7. Faber, W. R.., J. D. Bos, P. J. G. M. Rietra, H. Fas, and R. V. W. Van Eijk. 1983. Treponemicidal levels of amoxicillin in cerebrospinal fluid after oral administration. Sex. Transm. Dis. 10:148-150.
8. Garcia Monco, J. C., B. Fernandez Villar, and J. L. Benach. 1989. Adherence of Lyme disease spirochetes to glial cells and cells of glial
origin. J. Infect. Dis. 160:497.
9. Georgilis, K., M. Peacocke, and M. S. Klempner. 1992 Protection of the Lyme disease spirochete, B. burgdorferi from ceftriaxone by human skin fibroblasts, abstr. 165. p. A29. Program Abstr. 5th Int. Conf. Lyme Borreliosis. 1992.
10.Hardin, J. A., A. C. Steere, and S. E. Malawista. 1979. Immune complexes and the evolution of Lyme arthritis. Dissemination and localization of abnormal C1q binding activity. N. Engl. J. Med. 301:1358-1363.
11. Hassler, D., K, Riedel, J. Zorn, and V. Preac-Mursic. 1991. Pulsed high-dose cefotaxime therapy in refractory Lyme borreliosis. Lancet 338:193.
12. Haupl, T.. A. Krause, M. Rittig, C. Schoerner, J. R. Kalden, M. Simon, R. Wallich, and G. R. Burmester. 1992. Persistence of B. burgdorferi in chronic Lyme disease: altered immune regulation or evasion into immunologically privileged sites?. abstr. 149, p. A26. Program Abstr. 5th Int. Conf. Lyme Borreliosis. 1992.
13. Holden, C. 1992. Random samples: another gene therapy first. Science 256:1628.
14. Klempner, M. S., R. Noring, M. Peacocke, K. Georgilis, C. Braden, and R. A. Rogers. 1992. Invasion of human skin fibroblasts by the Lyme disease spirochete, B. burgdorferi. abstr. 164, p. A29. Program Abstr, 5th Int. Conf. Lyme Borreliosis, 1992.
15. Kuhn, T. S. 1963. The structure of scientific revolutions. University of Chicago Press, Chicago.
16. Liegner, K. B. 1990. Lyme disease. N. Engl. J. Med. 322:474-475.
17. Liegner, K. B. 1992. Minocycline in Lyme disease. J. Am. Acad. Dermatol. 26:263-264.
18. Liegner, K. B. 1993. Prevention of Lyme disease after tick bites. N. Engl. J. Med. 328:136-137.
19. Liegner, K. B. 1993. Minocycline in Lyme disease. J. Am.Acad. Dermatol. 28:131.
20. Liegner, K. B. A controlled trial of antimicrobial prophylaxis for Lyme disease after deer-tick bites. N. Engl. J. Med. in press.
21. Liegner, K. B., D. Dorward, and C. Garon. 1992. Lyme borreliosis (LB) studied with the Rocky Mountain Laboratory (RML) antigen-capture assay in urine. abstr. 104, p. A18. Program Abstr. 5th Int. Conf. Lyme Borreliosis,1992.
22. Liegner, K. B., C. E. Rosenkilde, G. L. Campbell, T. J. Quan, and D.
T. Dennis. 1992. Culture-confirmed treatment failure of cefotaxime and minocycline in a case of Lyme meningoencephalomyelitis in the United States, abstr. 63, p. A10. Program Abstr. 5th Int. Conf. Lyme Borreliosis,1992.
23. Liegner, K. B., and J. Selman. 1992. Global cerebellar atrophy in Lyme borreliosis. abstr. 55B, p. A10. Program Abstr. 5th Int. Conf. Lyme Borreliosis, 1992.
24. Liegner, K. B., J. R. Shapiro, D. Ramsay, A. J. Halperin, W. Hogrefe, and L. Kong. 1993. Recurrent erythema migrans despite extended antibiotic treatment with minocycline in a patient with persisting B. burgdorferi infection. J. Am. Acad. Dermatol. 28:312-314.
25. Ma, Y., A. Sturrock, and J. Weis. 1991. Intracellular localization of B. burgdorferi within endothelial cells., Infect. Immun. 59:671-678.
26. Mahmoud, A. A, F. 1992. The challenge of intracellular pathogens. N. Engl. J. Med. 326:761-762.
27. Mergny, J. L., G. Duval-Valentin, C. H. Nguyen, L. Perrouault, B. Faucon, M. Rougee, T. Montenay-Garestier, E. Bisagni, and C. Helene. 1992. Triple Helix-specific ligands. Science 256:1681-1983.
28. Merlo, A., B. Weder, E. Ketz, and L. Matter. 1989. Locked-in state in B. burdorferi meningitis. J. Neurol. 236:305-306.
29. Montgomery, R. R., M. H. Nathanson, and S. E. Malaista. 1992. The fate of B. burgdorferi in mouse macrophages: destruction, survival, recovery, abstr. 143, p. Ast Program Abstr. 5th Int. Conf. Lyme Borreliosis, 1992.
30. Preac-Mursic, V,, K. Weber, W. Pfister, B Wilske, B. Gross, A. Bauman, and J. Prokop. 1989. Survival of B. burgdorferi inantibiotically treated patients with Lyme borreliosis. Infection 17:355.
31. Roberts, R. W., and D. M. Crothers. 1992. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science 258:1463-1466.
32. Schutzer, S. E., P. K. Coyle, and M. Brunner. 1992. Specific serum immune complexes in Lyme disease. abstr. 135, p. A24. Program Abstr. 5th Int. Conf. Lyme Borreliosis, 1992.
33. Steere, A. C., E. Dwyer, and R. Winchester. 1990. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N. Engl. J. Med. 323:212-223.
34. Sullivan, P. 1992. Health insures limit drugs for Lyme disease. Sunday Star Ledger. Newark, N. J., volume 79 March 22. Section E. p. 1.
35. Szuromi, P. 1992. Triple-helix preference. Science 256:1607.
Liiteraturzitate
1. Brenner, C. 1992. Lyme disease. Asking the right questions. Science 257:1845.
JAMA 1992 Mar 11;267(10):1364-1367
Department of Medicine, SUNY at Stony Brook 11794-8153.
OBJECTIVE--To determine central nervous system (CNS) involvement in acutely disseminated Borrelia burgdorferi infection by measurement of borrelia-specific DNA using the polymerase chain-reaction (PCR) assay and to compare the results of this with standard serological tests. DESIGN--Prospective study with laboratory investigators blinded to clinical data. SETTING--Multicenter office practice with a central reference laboratory. PATIENTS--Cerebrospinal fluid (CSF) was collected from 12 patients with acute disseminated Lyme borreliosis with less than 2 weeks of active disease. The normal control specimens came from 16 patients whose CSF samples had been sent to the clinical laboratory for tests unrelated to the present study. MAIN OUTCOME MEASURES--Clinical evidence of disease and laboratory abnormalities. RESULTS--Eight of the 12 patients (four of six with multiple areas of erythema migrans and four of six with cranial neuritis without erythema migrans) had B burgdorferi-specific DNA in their CSF. Among the 12 patients studied, nine had acute cranial neuritis and six had multiple erythema migrans lesions. Just four of the eight who were found to have spirochetal DNA in their CSF had complaints suggestive of CNS infection. In three of the PCR-positive CSF samples, no other abnormalities were noted. None of 16 samples from controls were positive in the PCR assay. CONCLUSION--B burgdorferi can invade the CNS early in the course of infection. Careful consideration should be given to choosing antibiotics that achieve adequate CSF levels in patients with disseminated infection.Published erratum appears in JAMA 1992 Aug 19;268(7):872
Comments:
PMID: 1740859, UI: 92157287
Infect Immun 1991 Feb;59(2):671-8
Department of Pathology, University of Utah School of Medicine, Salt Lake City 84132.
The later stages of infection by the Lyme disease pathogen, Borrelia burgdorferi, are characterized by the persistence of the organism in individuals possessing a strong anti-Borrelia immune response. This suggests that the organism is sequestered in a tissue protected from the immune system of the host or there is a reservoir of the organism residing within the cells of the host. In this report, the ability of B. burgdorferi to gain entrance into human umbilical vein endothelial cells was explored as a model for invasion. Incubation of B. burgdorferi with human umbilical vein endothelial cells at ratios ranging from 200:1 to 5,000:1 resulted in the intracellular localization of 10 to 25% of B. burgdorferi in 24 h. The intracellular location of the spirochetes was demonstrated by the incorporation of radiolabeled B. burgdorferi into a trypsin-resistant compartment and was confirmed by double-immunofluorescence staining which differentiated intracellular from extracellular organisms. Actin-containing microfilaments were required for the intracellular localization, indicating that the host cell participates in the internalization process. Activation of endothelial cells by agents known to increase the expression of several adhesion molecules had no effect on the interaction of B. burgdorferi with the endothelial monolayer. This indicates that the endothelial receptor for B. burgdorferi is constitutively expressed and that internalization is not dependent upon adhesion molecules whose expression is induced by inflammatory mediators. The demonstration of B. burgdorferi within endothelial cells suggest that intracellular localization may be a potential mechanism by which the organism escapes from the immune response of the host and may contribute to persistence of the organism during the later stages of Lyme disease.
PMID: 1987083, UI: 91100043
Infect Immun 1993 Sep;61(9):3843-53
Department of Pathology, University of Utah School of Medicine, Salt Lake City 84132.
Sonicated Borrelia burgdorferi was previously reported to possess both B-cell mitogenic and interleukin-6 (IL-6) stimulatory activities. In this report, two outer surface lipoproteins, OspA and OspB, were purified from B. burgdorferi and assessed for the presence of these functions. OspA was purified from two strains, an OspB-deficient variant of HB19 and N40, while OspB was purified from the N40 strain. All lipoprotein preparations were free of endotoxin contamination, and polymyxin B failed to inhibit responses, indicating that media contamination was not contributing to biological assays. All three preparations were able to stimulate proliferation of mononuclear cells from naive C3H/HeJ and BALB/c mice. Depletion experiments indicated that the responding cells were B lymphocytes and not T lymphocytes. Purified OspA and OspB stimulated immunoglobulin M production by splenocyte cultures from naive mice, a property also previously attributed to sonicated B. burgdorferi. OspA and OspB also stimulated the production of IL-6 and tumor necrosis factor alpha by bone marrow-derived macrophages from BALB/c and C3H/HeJ mice. Cytokine production was enhanced by the presence of gamma interferon in the cultures, indicating that the magnitude of responses to these lipoproteins may be modulated by cytokines in the microenvironment of infected tissues. Human endothelial cells produced IL-6 when incubated with OspA and OspB, indicating that non-hematopoietic lineage cells can respond to the lipoproteins. Purified OspA and OspB had approximately equal activity, with responses detected in the range of 10 ng of lipoprotein per ml to 1 microgram of lipoprotein per ml. Comparison with published dose responses for lipoproteins purified from Escherichia coli indicates that OspA and OspB purified from B. burgdorferi are much more potent. The high potency of the B. burgdorferi lipoproteins and the ability of the spirochete to invade tissues and persist argue that they could be important in the localized events contributing to the pathology of Lyme disease.
PMID: 8359905, UI: 93366445
CRC Press, Boca Raton, Boston, London, New York, Washington D.C., 1993
Dr. Mattman's new method of culturing the spirochete was featured at this year's 10th Annual International Conference at the NIH in Bethesda, MD. Viewed as one of the most important discoveries presented at the Poster Session, Mattman's technique may be a new Gold Standard for determining spirochetal infections and persistent disease. Although her findings are compelling, Mattman feels she is up against skepticism in the medical community. Mattman feels this harkens back to other big breakthroughs, such as developing the vaccine for Whooping Cough and discovering the H. Pylori bacteria in ulcers. Because these discoveries challenged previous medical doctrine, the doctors were originally shunned for breaking away from "accepted facts". Mattman is confident that it is only a matter of time before her research will be given credibility
In explaining why current testing for Lyme disease is unreliable, Mattman referred to the blood test using immuno-fluorescence assay (IFA). Here, she explains, the lab is looking for the antibody. It won't always be detected, because the spirochete can "masquerade" in other forms that delude the immune response. Mattman explained that the spirochete like other bacteria, is not always in its classic form - there is much diversity in its appearance. This "diversity" in appearance is what is known as the "L Form" of the bacteria (named after this research at Lister Institute). In learning to recognize the "L Form" bacteria, Mattman has been able to culture spirochetes abundantly and profusely. For now, Dr. Mattman hopes the doctors will use a more reliable test other than IFA. She suggests using the polymerase chain reaction (PCR) test. This test picks up on the actual DNA of the spirochete. Mattman's lab has also worked for the University of Michigan, where she came face to face with L-forms of Meningitis and Rheumatoid Arthritis (RA). She emphasized that these also can be easily missed in the lab, because they are not always in the classic form with a "suit and bow tie".
Pointing to the screen, Mattman announced that we were the first audience to see a remarkable photo of Multiple Sclerosis spinal fluid mixed with red blood cells The red blood cells on the screen were filled with many spirochetes that were also seen emerging from the red blood cells. "We used to think that the red blood cells mainly transported gases through the body - now we know better," said Mattman. The spirochetes weren't only in the red blood cells, they PREFER the red blood cells. With this observation, Mattman feels that persistent infection could be attributed to the fact that antibiotics do not easily penetrate the red blood cell to target the spirochete.
Now that Dr. Mattman has been able to culture the spirochete, she is focusing on specific treatment. In recognizing that the spirochete can have numerous strains, she hopes to use cultured spirochetes for antibiotic sensitivity testing. This is already widely used for other bacterial infections. With this procedure, Mattman could find which antibiotic would work best for the individual patient. From a clinical perspective, this knowledge would give the treating physician an important edge in prescribing an appropriate antibiotic.
Mattman concluded that Lyme disease is as endemic here as Malaria is in the Tropics. She is convinced that, with the introduction of more reliable testing for LD, the figures will more accurately reflect the prevalence of Lyme disease.
From Kim Weber, Researcher Reveals Possible Lyme & Multiple Sclerosis Connection, Tick Talk May/June 1997
J Immunol 1993 Feb 1;150(3):909-15
Department of Internal Medicine, Yale University School of Medicine, New Haven, CT 06510.
The macrophage is a known reservoir for a number of infectious agents, and is therefore a likely candidate site for persistence of Borrelia burgdorferi, the Lyme spirochete. We report that unopsonized B. burgdorferi enter macrophages rapidly, resulting mainly in degradation but occasionally in apparent intracellular persistence. We studied uptake of spirochetes by macrophages by simultaneously labeling infected cells with antibodies to B. burgdorferi and with sequential components of the endocytic pathway, and we examined optical sections (0.5-1.0 micron in thickness) of these cells by confocal fluorescence microscopy at multiple time points after infection. We found that only 5 min of incubation at 37 degrees C were required for nearly 100% of B. burgdorferi to enter a lysosomal glycoprotein-positive compartment, whereas 60 min were required for 90% of the spirochetes to appear in a cathepsin L-positive compartment under the same conditions. We also labeled infected living cells with acridine orange to distinguish live from killed intracellular organisms. Although the large majority of spirochetes within a given cell were dead, we saw occasional live ones up to 24 h (the longest interval examined) after all extracellular organisms had been lysed in distilled water. Moreover, we can reculture spirochetes from macrophages after infection. Persistence of spirochetes within macrophages provides a possible pathogenetic mechanism for chronic or recurrent Lyme disease in man.
PMID: 8423346, UI: 93139523
Hum Pathol 1996 Oct;27(10):1025-34
Allergy-Immunology-Rheumatology Division, Department of Medicine, Faculty of Medicine, KhonKaen University, Thailand.
To perform the first systematic electronmicroscopic (EM) and immunoelectron microscopy (IEM) study of the pathological changes and the evidence of spirochete presence in synovial membranes and synovial fluid (SF) cells of patients with chronic Lyme arthritis. EM examination was performed on four synovial membrane and eight SF cell samples from eight patients with chronic Lyme disease. Spirochetal antigens in the samples were sought by IEM using monoclonal antibody to Borrelia burgdorferi outer surface protein A (OspA) as the immunoprobe. Prominent ultrastructural findings were surface fibrin-like material, thickened synovial lining cell layer and signs of vascular injury. Borrelia-like structures were identified in all four synovial membranes and in two of eight SF cell samples. The presence of spirochetal antigens was confirmed by IEM in all four samples studied (one synovial membrane and three SF cell samples). OspA labelling was in perivascular areas, deep synovial stroma among collagen bundles, and in vacuoles of fibroblasts in synovial membranes; and in cytophagosomes of mononuclear cells in SF cell samples. Electron microscopy adds further evidence for persistence of spirochetal antigens in the joint in chronic Lyme disease. Locations of spirochetes or spirochetal antigens both intracellulary and extracellulary in deep synovial connective tissue as reported here suggest sites at which spirochaetes may elude host immune response and antibiotic treatment.
PMID: 8892586, UI: 97047745
Am J Trop Med Hyg 1995 Feb;52(2):128-33
National Institute of Public Health, Department of Electron Microscopy, Prague, Czech Republic.
Lyme borreliosis is a newly recognized systemic infection with protean clinical manifestations. Because the localization of the causative spirochete (Borrelia burgdorferi) in infected tissues is unknown, we used electron microscopy to find spirochetes in the hearts of chronically infected mice. There were three predominant locations for the spirochete in the hearts. In mice infected for one month or less, the spirochetes were mostly in or around blood vessels. They were either in the lumen or in the perivascular space. Mice infected for more than one month had B. burgdorferi in cardiac myocytes as well, often with clear spaces around them. The third area in which spirochetes were common was collagen fibers; the borreliae were wrapped around fibers with their long axis parallel to the fibers. The number of spirochetes was relatively low, but there was no appreciable decrease in numbers of spirochetes with increasing time postinfection. Inflammatory infiltrates were primarily in the endocardium and pericardium, but spirochetes were generally not in or near areas of inflammation. These data are consistent with previously published information that have identified the heart as a site of chronic infection and inflammation in the mouse. The studies extend our understanding of the behavior of the spirochete in vivo by identifying common locations of B. burgdorferi and by noting the disparity between infection and inflammation.
PMID: 7872439, UI: 95177294
Infection 1998 Nov-Dec;26(6):364-7
Greenwich Hospital, CT 06830, USA.
[Medline record in process]
Since culture of Borrelia burgdorferi from patients with chronic Lyme disease has been an extraordinarily rare event, clarification of the nature of the illness and proving its etiology as infectious have been difficult. A method for reliably and reproducibly culturing B. burgdorferi from the blood of patients with chronic Lyme disease was therefore sought by making a controlled blood culture trial studying 47 patients with chronic Lyme disease. All had relapsed after long-term oral and intravenous antibiotics. 23 patients with other chronic illness formed the control group. Positive cultures were confirmed by fluorescent antibody immuno-electron microscopy using monoclonal antibody directed against Osp A, and Osp A PCR. 43/47 patients (91%) cultured positive. 23/23 controls (100%) cultured negative. Although persistent infection has been, to date, strongly suggested in chronic Lyme disease by positive PCR and antigen capture, there are major problems with these tests. This new method for culturing B. burgdorferi from patients with chronic Lyme disease certainly defines the nature of the illness and establishes that it is of chronic infectious etiology. This discovery should help to reestablish the gold standard in laboratory diagnosis of Lyme disease.
PMID: 9861561, UI: 99078554
J Clin Neuroophthalmol 1993 Sep;13(3):155-61; discussion 162
Max-von-Pettenkofer-Institut für Hygiene und Medizinische Mikrobiologie, Ludwig-Maximilian-Universität München, Germany. The persistence of Borrelia burgdorferi in six patients is described. Borrelia burgdorferi has been cultivated from iris biopsy, skin biopsy, and cerebrospinal fluid also after antibiotic therapy for Lyme borreliosis. Lyme Serology: IgG antibodies to B. burgdorferi were positive, IgM negative in four patients&; in two patients both IgM and IgG were negative. Antibiotic therapy may abrogate the antibody response to the infection as shown by our results. Patients may have subclinical or clinical disease without diagnostic antibody titers. Persistence of B. burgdorferi cannot be excluded when the serum is negative for antibodies against it.
PMID: 8106639, UI: 94149159
Infection 1996 Jan-Feb;24(1):9-16
Max v. Pettenkofer Institut, Ludwig-Maximilians-Universitat Munchen, Germany. For a better understanding of the persistence of Borrelia burgdorferi sensu lato (s. l. ) after antibiotic therapy the kinetics of killing B. burgdorferi s. l. under amoxicillin, doxycycline, cefotaxime, ceftriaxone, azithromycin and penicillin G were determined. The killing effect was investigated in MKP medium and human serum during a 72 h exposure to antibiotics. Twenty clinical isolates were used, including ten strains of Borrelia afzelii and ten strains of Borrelia garinii. The results show that the kinetics of killing borreliae differ from antibiotic to antibiotic. The killing rate of a given antibiotic is less dependent on the concentration of the antibiotic than on the reaction time. Furthermore, the data show that the strains of B. afzelii and B. garinii have a different reaction to antibiotics used in the treatment of Lyme borreliosis and that different reactions to given antibiotics also exist within one species. The B. garinii strains appear to be more sensitive to antibiotics used in therapy. Furthermore, the persistence of B. burgdorferi s. l. and clinical recurrences in patients despite seemingly adequate antibiotic treatment is described. The patients had clinical disease with or without diagnostic antibody titers to B. burgdorferi. Published erratum appears in Infection 1996 Mar-Apr;24(2):169 PMID: 8852456, UI: 97005145
Infection 1989 Nov-Dec;17(6):355-9
Neurologische Klinik Grosshadern, Munchen, FR Germany. The persistence of Borrelia burgdorferi in patients treated with antibiotics is described. The diagnosis of Lyme disease is based on clinical symptoms, epidemiology and specific IgG and IgM antibody titers to B. burgdorferi in serum. Antibiotic therapy may abrogate the antibody response to the infection as shown in our patients. B. burgdorferi may persist as shown by positive culture in MKP-medium; patients may have subclinical or clinical disease without diagnostic antibody titers to B. burgdorferi. We conclude that early stage of the disease as well as chronic Lyme disease with persistence of B. burgdorferi after antibiotic therapy cannot be excluded when the serum is negative for antibodies against B. burgdorferi. PMID: 2613324, UI: 90129322
Infection 1996 May-Jun;24(3):218-26 Max von Pettenkofer-Institut, Ludwig-Maximilians-Universitat Munchen, Germany. As clinical persistence of Borrelia burgdorferi in patients with active Lyme borreliosis occurs despite obviously adequate antibiotic therapy, in vitro investigations of morphological variants and atypical forms of B. burgdorferi were undertaken. In an attempt to learn more about the variation of B. burgdorferi and the role of atypical forms in Lyme borreliosis, borreliae isolated from antibiotically treated and untreated patients with the clinical diagnosis of definite and probable Lyme borreliosis and from patient specimens contaminated with bacteria were investigated. Furthermore, the degeneration of the isolates during exposure to penicillin G in vitro was analysed. Morphological analysis by darkfield microscopy and scanning electron microscopy revealed diverse alterations. Persisters isolated from a great number of patients (60-80%) after treatment with antibiotics had an atypical form. The morphological alterations in culture with penicillin G developed gradually and increased with duration of incubation. Pleomorphism, the presence of elongated forms and spherical structures, the inability of cells to replicate, the long period of adaptation to growth in MKP-medium and the mycoplasma-like colonies after growth in solid medium (PMR agar) suggest that B. burgdorferi produce spheroplast-L-form variants. With regard to the polyphasic course of Lyme borreliosis, these forms without cell walls can be a possible reason why Borrelia survive in the organism for a long time (probably with all beta-lactam antibiotics) [corrected] and the cell-wall-dependent antibody titers disappear and emerge after reversion. Published erratum appears in Infection 1996 Jul-Aug;24(4):335 PMID: 8811359, UI: 96407306
J Clin Invest 1991 Jul;88(1):82-92
Department of Medicine, University of Texas Health Science Center, San Antonio 78284.
A nonmotile mutant of Borrelia burgdorferi, the etiologic agent of Lyme
disease, was isolated and characterized. The mutant was compared with the
wild-type predecessor as well as with a motile back-revertant of the same
genetic background. The mutant lacked, by morphologic, biochemical, and
immunologic criteria, the major structural protein of flagella, flagellin.
This mutation was not associated with major DNA rearrangements or with
failure of transcription. An apparent consequence of a loss of flagella was
reduced ability to penetrate human endothelial cell layers in vitro. In
another assessment of functional significance, the flagella-less mutant was
equal if not superior to flagella-bearing, isogenic isolates when examined
in an enzyme-linked immunosorbent assay for anti-B. burgdorferi antibodies
in the sera of Lyme disease patients. These studies of a mutant, the first
among pathogenic Borrelia spp. to be characterized, indicate that the
flagellum and motility it confers play a role in B. burgdorferi's invasion
of human tissues. A flagella-less B. burgdorferi may be useful as the basis
of a more specific immunoassay and a vaccine for protection against Lyme
disease.
PMID: 2056133, UI: 91277311
Infect Immun 1995 Apr;63(4):1573-1580 Department of Microbiology and Medicine, University of Texas Health Science Center at San Antonio 78284. All Borrelia burgdorferi sensu lato isolates characterized to date have one or a combination of several major outer surface proteins (Osps). Mutants of B. burgdorferi lacking Osps were selected with polyclonal or monoclonal antibodies at a frequency of 10(-6) to 10(-5). One mutant that lacked OspA, -B, -C, and -D was further characterized. It was distinguished from the OspA+B+ cells by its (i) autoaggregation and slower growth rate, (ii) decreased plating efficiency on solid medium, (iii) serum and complement sensitivity, and (iv) diminished capacity to adhere to human umbilical vein endothelial cells. The Osp-less mutant was unable to evoke a detectable immune response after intradermal live cell immunization even though mutant survived in mouse skin for the same duration as wild-type cells. Polyclonal mouse serum raised against Osp-less cells inhibited growth of the mutant but not of wild-type cells, an indication that other antigens are present on the surface of the Osp-less mutant. Two types of monoclonal antibodies (MAbs) with growth-inhibiting properties for mutant cells were identified. The first type bound to a 13-kDa surface protein of B. burgdorferi sensu stricto and of B. afzelii. The MIC of the Fab fragment of one MAb of this type was 0. 2 micrograms/ml. The second type of MAb to the Osp-less mutant did not bind to B. burgdorferi components by Western blotting (immunoblotting) but did not bind to unfixed, viable cells in immunofluorescence and growth inhibition assays. These studies revealed possible functions Osp proteins in borrelias, specifically serum resistance, and indicated that in the absence of Osp proteins, other antigens are expressed or become accessible at the cell surface. PMID: 7890424, UI: 95197293
J Clin Invest 1994 Jul;94(1):454-457
Department of Medicine, University of Medicine and Dentistry of New Jersey-New Jersey Medical School, Newark 07103.
Borrelia burgdorferi (Bb), the cause of Lyme disease, has appeared not to evoke a detectable specific antibody response in humans until long after infection. This delayed response has been a biologic puzzle and has hampered early diagnosis. Antibody to the abundant organism-specific outer surface proteins, such as the 31-kD OspA, has rarely been detected less than 6 mo after infection. Antibody to a less organism-specific 41-kD flagellin protein, sharing common determinants with other bacteria and thus limiting its diagnostic potential, may appear after 4 to 6 wks. To investigate our hypothesis that specific antibody to OspA may actually be formed early but remain at low levels or bound in immune complexes, we analyzed serum samples from patients with concurrent erythema migrans (EM). This is the earliest sign of Lyme disease and occurs in 60-70% of patients, generally 4-14 d after infection. We used less conventional but more sensitive methods: biotin-avidin Western blots and immune complex dissociation techniques. Antibody specificity was confirmed with recombinant OspA. Specific complexed antibody to whole Bb and recombinant OspA was detected in 10 of 11 of the EM patients compared to 0 of 20 endemic area controls. IgM was the predominant isotype to OspA in these EM patients. Free IgM to OspA was found in half the EM cases. IgM to OspA was also detected in 10 of 10 European patients with EM who also had reactive T cells to recombinant OspA. In conclusion a specific antibody response to OspA occurs early in Lyme disease. This is likely to have diagnostic implications.
PMID: 8040289, UI: 94314934
WH Freeman and Co, New York, 1997.
University of California Medical Center, San Francisco, CA, USA
TI-1 Antigene = Thymus-unabhängige Antigene vom Typ 1. Sie hinterlassen im Immunsystem keinen bleibenden Eindruck (keinen sog. "memory effect"), der die Immunabwehr beim zweiten Eintreffen der Antigene in unserem Körper beschleunigen und erhöhen könnte . Beispiele: Zellwandbestandteile (z.B. Osp's) und Bestandteile der äußeren Oberfläche (z.B. LPS) von Bb (siehe Fig. 1).
Cell 1997 Apr 18;89(2):275-285
Department of Pathology and Laboratory Medicine, University of Texas Medical School at Houston, 77030,
USA.
Wir haben ein komplexes genetisches System in der Lyme-Borreliose-Spirochäte Borrelia burgdorferi identifiziert und charakterisiert, das einer umfassenden Anitgenvariation eines Oberflächen-Lipoproteins, VlsE (vls expression site, vls = vmp-like sequence, vmp = variable major protein), dient. Wir fanden, daß ein 28 kb ausgedehntes lineares Plasmid der Borrelia burgdorferi B31 (lp28-1) eine vmp-ähnliche Sequenzstelle (vls) enthält, das sehr dem variablen Hauptprotein-System (vmp) für Antigenvariation des Rückfallfiebers gleicht. Teile von etlichen der 15 nicht-dargestellten (nicht-ausgeprägten, stummen) vls-Kassetten-Sequenzen oberhalb einer vls-Ausprägungsposition (Expressionsposition) rekombinierten in die zentrale vls-Expressionspositions-Kassettengegend während der Infektion von C3H/HeN-Mäuse, was zur Antigenvariation des dadurch erzeugten Lipoproteins führte. Diese kombinatorische Variation hätte die Möglichkeit, Millionen von Antigenvariationen im Säugetier-Wirt hervorzubringen (Übersetzung des Abstracts: J.G.).
An epitope is an antigenic determinant, or a site on the surface of an antigenic molecule, to which a single antibody binds. Epitope spreading (ES) refers to the development of an immune response to epitopes distinct from, and noncross-reactive with, the disease-causing epitope. Diversification, or the ability of the immune system to attack multiple targets on a pathogen has obvious advantages. Here we review some of the evidence regarding its role in autoimmunity, in humans and in animal disease models. We consider the implications of ES on the development of highly specific therapies for autoimmune disease. We stress that pathogenic ES probably occurs in the context of inherent abnormalities in control mechanisms for the prevention of autoimmunity or other genetic predisposing factors.
Nat Rev Immunol. 2002 Feb;2(2):85-95.
Evidence continues to accumulate supporting the hypothesis that tissue damage during an immune response can lead to the priming of self-reactive T and/or B lymphocytes, regardless of the specificity of the initial insult. This review will focus primarily on epitope spreading at the T-cell level. Understanding the cellular and molecular basis of epitope spreading in various chronic immune-mediated human diseases and their animal models is crucial to understanding the pathogenesis of these diseases and to the ultimate goal of designing antigen-specific treatments.
Adv Virus Res. 2001;56:199-217.
Epidemiological studies indicate that host immunogenetics and history of infection, particularly by viruses, may be a necessary cofactor for the induction of a variety of autoimmune diseases. To date, however, there is no clear-cut evidence, either in experimental animal models or in human autoimmune disease, that supports either molecular mimicry (Wucherpfennig and Strominger, 1995; Fujinami and Oldstone, 1985) or a role for superantigens (Scherer et al., 1993) in the initiation of T cell-mediated autoimmunity. In contrast, the current data provide compelling evidence in support of a major role for epitope spreading in the induction of myelin-specific autoimmunity in mice persistently infected with TMEV. It is significant that two picornaviruses closely related to TMEV, coxsackievirus (Rose and Hill, 1996) and encephalomyocarditis virus (EMCV) (Kyu et al., 1992), have been similarly shown to persist (either the viral RNA or the infectious virus) in their target organs and have been associated with the development of chronic autoimmune diseases, including myocarditis and diabetes. Thus, inflammatory responses induced by viruses that trigger proinflammatory Th1 responses, and have the ability to persist in genetically susceptible hosts, may lead to chronic organ-specific autoimmune disease via epitope spreading. Epitope spreading has important implications for the design of antigen-specific therapies for the potential treatment of MS and other autoimmune diseases. This process indicates that autoimmune diseases are evolving entities and that the specificity of the effector autoantigen-specific T cells varies during the chronic disease process. Our experiments employing tolerance in R-EAE clearly indicate that antigen-specific treatment of ongoing disease is possible for preventing disease relapses, provided the proper relapse-associated epitope is targeted (Vanderlugt et al., 1999). However, the ability to identify relapse-associated epitopes in humans will be a difficult task because immunodominance will vary in every individual. The use of costimulatory antagonists that can induce anergy without requiring prior knowledge of the exact epitopes (Miller et al., 1995b), or the use of therapies that induce bystander suppression (Nicholson et al., 1997; Brocke et al., 1996), may thus be more practical current alternative therapies for the treatment of human autoimmune disease.
Immunol Rev. 1998 Aug;164:231-9.
A complex interplay of cells, soluble macromolecules, and antigen lead to a productive immune response that evolved for the survival of species. While the immune system is intended to protect from foreign agents, such as bacterial and viral infection, the presence of autoimmune diseases indicates that the system is not perfect in differentiating antigen that may cause harm from benign self constituents. The concept of epitope spreading, where many determinants on an offending antigen are the focus of immune attack, is an efficient means of clearing an infectious agent. However, the same mechanisms that lead to a diverse immune response may be harmful when the targets of attack are self tissues or self macromolecules. This review will examine the forms of self antigens that may initiate autoimmunity and the potential role of B lymphocytes, as autoantigen-presenting cells, as one mechanism by which diversification of autoimmunity may occur.
Immunol Rev. 1998 Aug;164:185-200.
How the immune response matures from recognizing a single or a few structures of the antigen to many is an obviously important process. Models of B-cell epitope spreading have been developed in a variety of systems. For example, immunization of animals with PPPGMRPP, one of the earliest B-cell epitopes in the anti-Sm response found in human lupus, leads to antispliceosomal autoimmunity and features of lupus. The humoral immune response spreads from PPPGMRPP to other structures of the spliceosome in an apparently reproducible sequence. B-cell epitope spreading has provided the experimental basis from which a relationship between lupus and Epstein-Barr virus was suspected. An understanding of B-cell epitope spreading is likely to lead to important principles in basic immunology and to answers to human disease problems.
Immunol Rev. 1998 Aug;164:63-72.
Epitope spreading is a process whereby epitopes distinct from and non-cross-reactive with an inducing epitope become major targets of an ongoing immune response. This phenomenon has been defined in experimental and natural situations as a consequence of acute or persistent infection and secondary to chronic tissue destruction that occurs during progressive autoimmune disease. We have investigated the functional significance of this process in the chronic stages of both autoimmune and virus-induced central nervous system (CNS) demyelinating disease models in the SJL/J mouse. During the relapsing-remitting course of experimental autoimmune encephalomyelitis (R-EAE) induced with defined encephalitogenic myelin peptides, CD4+ T cells specific for endogenous epitopes on both the initiating myelin protein (intramolecular epitope spreading) and distinct myelin proteins (intermolecular epitope spreading) are primed secondary to myelin destruction during acute disease and play a major functional role in mediating disease relapses. Similarly, epitope spreading to endogenous myelin epitopes appears to play a major functional role in the chronic-progressive course of Theiler's murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD), a virus-induced CD4+ T-cell-mediated immunopathology. In TMEV-IDD, myelin destruction is initiated by virus-specific CD4+ T cells which target virus epitopes persisting in CNS-derived antigen-presenting cells. However, the chronic stage of this progressive disease is associated with the activation of CD4+ T cells specific for multiple myelin epitopes. In both models, the temporal course of T-cell activation occurs in a hierarchical order of epitope dominance, spreading first to the most immunodominant epitope and progressing to lesser immunodominant epitopes. In addition, epitope spreading in R-EAE is regulated predominantly by CD28/B7-1 co-stimulatory interactions, as antagonism of B7-1-mediated co-stimulation using anti-B7-1 F(ab) fragments is an effective ameliorative therapy for ongoing disease. The process of epitope spreading has obvious important implications for the design of antigen-specific therapies for the treatment of autoimmune disease since these therapies will have to identify and target endogenous self epitopes associated with chronic tissue destruction.
First isolation of Borrelia burgdorferi from an iris biopsy.
Preac-Mursic V, Pfister HW, Spiegel H, Burk R, Wilske B, Reinhardt S, Bohmer R
Kill kinetics of Borrelia burgdorferi and bacterial findings in relation to the treatment of Lyme borreliosis.
Preac Mursic V, Marget W, Busch U, Pleterski Rigler D, Hagl S
Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis.
Preac-Mursic V, Weber K, Pfister HW, Wilske B, Gross B, Baumann A, Prokop J
Formation and cultivation of Borrelia burgdorferi spheroplast-L-form variants.
Mursic VP, Wanner G, Reinhardt S, Wilske B, Busch U, Marget W
A flagella-less mutant of Borrelia burgdorferi. Structural, molecular, and in vitro functional characterization.
Sadziene A, Thomas DD, Bundoc VG, Holt SC, Barbour AG
Borrelia burgdorferi mutant lacking Osp: biological and immunological characterization.
Sadziene A, Thomas DD, Barbour AG
Early and specific antibody response to OspA in Lyme Disease.
Schutzer SE, Coyle PK, Dunn JJ, Luft BJ, Brunner M
Immunology
3rd EditionKuby J
Antigenic variation in Lyme disease borreliae by promiscuous
recombination of VMP-like sequence cassettes.
Zhang JR, Hardham JM, Barbour AG, Norris SJ
We have identified and characterized an elaborate genetic system in the Lyme disease spirochete Borrelia burgdorferi that promotes extensive antigenic variation of a surface-exposed lipoprotein, VlsE. A 28 kb linear plasmid of B. burgdorferi B31 (lp28-1) was found to contain a vmp-like sequence (vls) locus that closely resembles the variable major protein (vmp) system for antigenic variation of relapsing fever organisms. Portions of several of the 15 nonexpressed (silent) vls cassette sequences located upstream of vlsE recombined into the central vlsE cassette region during infection of C3H/HeN mice, resulting in antigenic variation of the expressed lipoprotein. This combinatorial variation could potentially produce millions of antigenic variants in the mammalian host.
EPITOPE SPREADING
Clin Exp Dermatol. 2001 Jul;26(5):427-33.
Epitope spreading: protection from pathogens, but propagation of autoimmunity?
Powell AM, Black MM.
St John's Institute of Dermatology, St Thomas' Hospital, London, UK.
ami.powell@kcl.ac.uk
Epitope spreading in immune-mediated diseases: implications for immunotherapy.
Vanderlugt CL, Miller SD.
Department of Microbiology-Immunology, Interdepartmental Immunobiology Center, Northwestern University Medical School, 303 E. Chicago Avenue, Chicago, IL 60611, USA.
Virus-induced autoimmunity: epitope spreading to myelin autoepitopes in Theiler's virus infection of the central nervous system.
Miller SD, Katz-Levy Y, Neville KL, Vanderlugt CL.
Department of Microbiology-Immunology and Interdepartmental Immunobiology Center, Northwestern University Medical School, Chicago, Illinois 60611, USA.
Epitope spreading: the role of self peptides and autoantigen processing by B lymphocytes.
Mamula MJ.
Yale University School of Medicine, New Haven, CT 06520-8031, USA.
mark.mamula@yale.edu
B-cell epitope spreading in autoimmunity.
James JA, Harley JB.
Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma Medical Research Foundation, Oklahoma City 73104, USA.
The functional significance of epitope spreading and its regulation by co-stimulatory molecules.
Vanderlugt CL, Begolka WS, Neville KL, Katz-Levy Y, Howard LM, Eagar TN, Bluestone JA, Miller SD.
Department of Microbiology-Immunology, Northwestern University Medical School, Chicago, Illinois 60611, USA.
version: February 1, 2008
Address of this Page
Home