Home -- Symptoms -- Cycles -- Compartment Models Displaying Lyme Disease Symptom Cycles
The immune system responds to what I will abbreviate as toxins such as
Via molecular mimicry, also autoimmune processes can be triggered by Bb proteins (Sigal 1997, Sigal and Williams 1997, Hemmer et al.1999, Klempner et al. 1999, an ongoing study headed by Adriana Marques, Laboratory of Clinical Investigation, National Institute of Allergy and Infectious Diseases, reported in NIAID's News). T-cell subpopulations (of short-lived T-cells) responsible for autoimmune processes might persist as long as sufficient levels of such proteins are present in the host (Kuby, chapter 12, S. 305). The existence of such autoimmune processes could bring about a decoupling of infection and inflammation, both in space and time. If such processes support e.g. symptom cycles, their period may differ from the periods of cycles triggered by infectious processes. The following description refers to an immune response directed against an infection.
The immune response should be visualized as being twofold:
The immune response ends when
These two steps are combined into a feedback control process aiming at the elimination of the toxin. Unlike with many other infections, the incubation time of the toxins is so short (i.e. some hours, like in viral influenza) that the immune system's memory is irrelevant (pp. 202, 447 in Kuby, 1997). Thus, steps 1 and 2 will be repeated in much the same form as long as the niches release new toxins into compartments under immune system surveillance. The feedback control system is locked into undamped oscillations.
As is illustrated in Fig. 10, the basic building blocks of the immune response model are
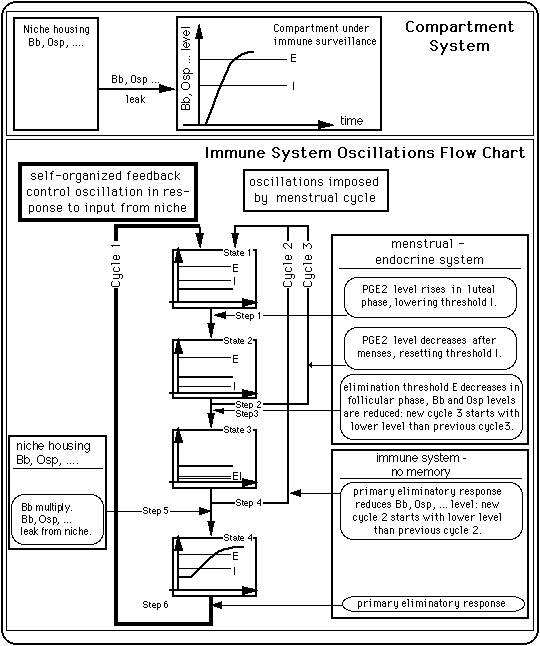
Fig. 10: A simple compartment system and an immune system control scheme that produces oscillations between an inflamed state and a symptom free state.
Sections V. 1 and V. 2 will give simple examples of possible feedback control cycles (cycles of type 1). The mechanism driving the cycles in the absence of antibiotics are different from the one responsible for cycles under the influence of antibiotics.
Fig. 11 shows a compartment model and the symptom cycles produced by an oscillatory immune system. The concentration C(t) of the substance invoking immune response is assumed to be proportional to the Bb concentration.
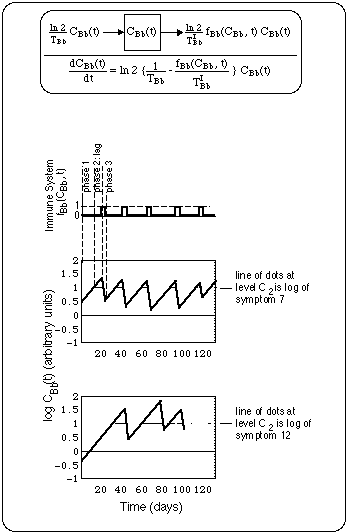
Fig. 11: Schematic of flare cycles driven by oscillatory immune response fBb(CBb(t), t), where
Note the logarithmic concentration scale in diagrams for C(t): A straight line up (down) represents exponential growth (decay).
Immune system always starts up (f = 1) when Bb concentration has reached a concentration C1. Thus, the immune system being triggered by Bb concentration, always lags behind Bb growth.
Immune system always shuts down (f = 0) at Bb concentration C2, i.e. before all Bb have been eliminated. To simplify the figure, thresholds C1 and C2 have been assumed to coincide.
f has been chosen symptom specific, assuming that immune system has localized properties. f's are chosen such that logs of symptoms 7 and 12 are reproduced (see symptom logs placed at level C2).
Data used in calculations for illustration purposes
The phases of a flare cycle are:
In Fig. 11 we have fitted the compartment model to the symptom cycles by allowing a variation of the location of the peaks of the immune system switching function fBb(CBb(t), t), while keeping the Bb generation time TBb and the elimination half life TIBb fixed (thus the widths of the peaks are constant). This results in shifting fixed zig-zag segments (one branch going up the other going down) around. We did not succeed fitting the data of a symptom log by doing the reverse, i.e. keeping the f-curve fixed while adjusting the slopes of the individual zig-zag branches. Thus, it seems that the times when the immune system loses track of a Bb population and starts seeing the next one are variable.
The geometry of the curves in Fig. 11 lets us see the following properties of symptom cycles before antibiotic treatment:
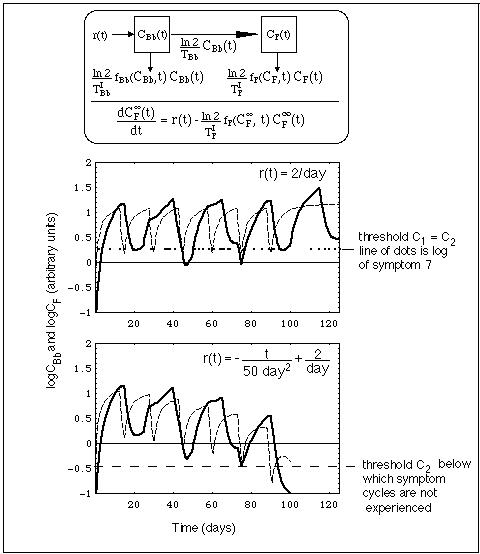
Fig. 12: Concentration CBb(t) of Bb population outside niche (dashed line) and CF(t) of Bb fragments (heavy line) resulting from a Bb source r(t). Concentrations are calculated with compartment model shown in top of figure.
Superimposed on the concentration curves in the upper diagram at level C2 is a section of the symptom log of symptom 7, i.e. the vertical series of dots for symptom 7 (Light Hypersentisitivity) between day 272 and 292 in Fig. 1.
Like in Fig. 11, f is the immune switching function, while C1 and C2 are the immune response start up and shut down thresholds, respectively.
In the case depicted in Fig. 12,
Data used in computations
TBb = 5 days.
TIBb = 0.5 days.
TIF = 1 day.
r(t) as stated in upper right corner of diagrams.
Specific properties of this system are:
Thus, a Bb populations entering the system from niches drive flare cycles, much like dust entering into a room from an outside source makes periodic room cleaning necessary. As long as there exists the dust source outside, we need to periodically clean the room. Similarly, the Bb niche population is called "active" by J.J. Burrascano as long as the Bb fragment concentration oscillates across the threshold for a Herxheimer reaction, CF(t) > C2.
The model explains an interesting feature consistent with that analogy:
When the fragment concentration exceeds a threshold C2, this induces an (Herxheimer-like) immune reaction accompanying the fragment clean-up (Hurley 1992). The reaction subsides -as does the clean-up- once the fragment concentration has fallen below some other threshold (hysteresis). The relationship between C2 and the immune response (cytokine production) is highly non-linear, including a lag phase at the beginning (Frieling et al. 1997).
Only when the cell wall antibiotic is present at the minimum inhibitory concentration or some small integer multiple of it, the Bb pool cannot grow exponentially, not even in the absence of an immune response. When the antibiotic concentration is subinhibitory allowing the Bb population to grow exponentially, the symptoms worsen and their temporal development reflects this (see Fig. 11). Since during the time of the treatment with cefuroxime the patient experienced this worsening (see Fig. 8), we have a strong indication that active Bb have survived the 30 days of ceftriaxone and subsequent 30 days of doxycycline treatment (days 99 - 166).
The minima of the fragment compartment concentrations oscillations follow roughly an asymptotic curve, the differential equation of which is given in the box in the top of Fig. 12. The theoretical background is given in the Notation Section. Fig. 12 shows that the Bb concentration oscillates between two equilibrium levels. Only if these tend towards zero, will the infection be cured. For that the source of Bb must eventually be stopped (r(t) -> 0, see lower diagram in Fig. 12), which demands an elimination of the niche population.
When the immune responses switching functions fBb and fF do not have the same frequency, the fragment concentration, being driven by the Bb concentration, shows a beat-like behavior in time. Correspondingly, the time during which this concentration is above a threshold level C2 shows these beats, too. This provides a simple interpretation of apparently irregular symptom logs (probably there are more involved reasons for these irregularities, including spontaneous and induced statistical fluctuations in the host's immune system). As an illustration, the log of symptom 7 is superimposed on the beats of CF(t). The log's irregularities are roughly reproduced by the modeled beats, and the fit could be improved by further shifting the periods of the two immune response functions.
The method is exemplified with the data of a female patient's symptom log. Specifics of this log are:
The models use as input
The results of the model seem to be stable against reasonable variations of input parameters (2), but their relative contributions, i.e. the effect of the immune system relative to the antibiotic (expressed as arrows in Fig. 12), needs to be discussed further. Once the immune system switching function f thresholds can be deduced from medico-microbiological principles, therfore not needing to be adapted to get the modeled symptom logs fit the data (as done in this analysis), the presented -rather empirical- interpretation of the infection's flare cycles would be markedly improved.
Ball P, The self-made tapestry: pattern formation in nature, Oxford University Press, Oxford, New York, Tokyo, 1999.
Barkley M, Harris N, Szantyr B, 1997. The Lyme Urine Antigen Test (LUAT) during antibiotic therapy in women with recurrent menstrual cycles. 10th Annual Int. Conf. on Lyme Borreliosis, National Institute of Health, Bethesda, MD, April 28-30.
Beck G, Habicht GS, Benach JL, Coleman JL, Lysik RM, O'Brien RF, A role for interleukin-1 in the pathogenesis of Lyme disease. Zentralbl Bakteriol Mikrobiol Hyg [A] 263(1-2):133-6 , 1986.
Bingen E, Goury V, Bennani H, Lambert-Zechovsky N, Aujard Y, Darbord JC, Bactericidal activity of beta-lactams and amikacin against Haemophilus influenzae: effect on endotoxin release. J. Antimicrob. Chemother. 30:165-172, 1992.
Bleiweiss JD, When to Suspect Lyme Disease?
Bransfield RC, Sex & Lyme.
Bristol-Myers Squibb, Pharmaceutical Information, 777 Scudders Mill Road Plainsboro, NJ 08536, Tel.: +1 (609) 252-4000 or (800) 332 - 2056.
Brorson O, Brorson SH, In vitro conversion of Borrelia burgdorferi to cystic forms in spinal fluid and transformation to mobile spirochetes by incubation in BSK-H medium. Infection 26:144-150, 1998.
Bericht des Landeshygiene-Instituts Mecklenburg-Vorpommern, Schloßstraße 8, D - 17235 Neustrelitz, Tel. + 49 -3981- 272 - 100 (Dr. Kober), 1997.
Burns MJ, Furie MB, Borrelia burgdorferi and Interleukin-1 Promote the Transendothelial Migration of Monocytes In Vitro by Different Mechanisms, Infect Immun 66: 4875-4883, 1998.
Burrascano JJ, The new Lyme disease: diagnostic hints and treatment guidelines for tick-borne illnesses.
Buxton Hopkin DA., Too rapid destruction of gram-negative organisms. Lancet i:603-604, 1977.
Chambers HF, Hadley WK, Jawetz E, "Beta-lactam antibiotics & other inhibitors of cell wall synthesis", pages 725-726 in: Basic & Clinical Pharmacology (Katzung BG, ed.), Appleton & Lange, Stamford, CT, USA, 1998.
Cohen J, and McConnell JS, Antibiotic-induced endotoxin release. Lancet i:1089-1090, 1985. (Letter).
Cohen, J, and McConnell JS, Release of endotoxin from bacteria exposed to ciprofloxacin and its prevention with polymixin B. Eur. J. Clin. Microbiol. 5:13-17, 1986.
Crosby HA, Bion JF, Penn CW, and Elliott TSJ, Antibiotic-induced release of endotoxin from bacteria in vitro. J. Med. Microbiol. 40:23-30, 1994.
Coyle, PK, Borrelia burgdorferi infection: clinical diagnostic techniques. Immunol Invest 1997 Jan; 26(1&2):117-128
Damas P Ledoux D, Nys M , Vrindts Y, De Groote D, Franchimont P, and Lamy M., Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann. Surg. 215:356Ð362, 1992.
De Boer RJ, Perelson AS, Kevrekidis IG, Immune network behavior--I. From stationary states to limit cycle oscillations., Bull Math Biol 55(4):745-80, 1993.
Dever LL, Torigian CV, and Barbour AG, In Vitro activities of the everninomicin SCH 27899Êand other newer antimicrobial agents against Borrelia burgdorferi, Antimicrob Agents Chemother. 43(7): 1773-17, 1993.
Dibrov BF, Livshits MA, Vol'kenshtein MV, Mathematical model of the immune response, Biofizika 1976 Sep-Oct;21(5):905-9, 1976.
Dibrov BF, Livshits MA, Vol'kenshtein MV, Mathematical model of the immune response. IV. Threshold character of the infectious process, Biofizika 1978 Jan-Feb;23(1):143-7, 1978.
Dotevall L, Alestig K, Hanner P, Norkrans G, Hagberg L, The use of doxycycline in nervous system Borrelia burgdorferi infection. Scand J Infect Dis Suppl 1988;53:74-9.
Dotevall L, Hagberg L, Penetration of doxycycline into cerebrospinal fluid in patients treated for suspected Lyme neuroborreliosis. Antimicrob Agents Chemother 1989 Jul;33(7):1078-80.
Eng RHK, Smith SM, Fan-Havard P, and Ogbara T, 1993. Effect of antibiotics on endotoxin release from gram-negative bacteria. Diagn. Microbiol. Infect. Dis. 16:185-189.
Evans ME, Pollack M, Effect of antibiotic class and concentration on the release of lipopolysaccharide from Escherichia coli. J. Infect. Dis. 167:1336-1343, 1993.
Fallon BA, Nields JA, Microbiology of Borrelia burgdorferi, in: "Lyme Disease: A Neuropsychiatric Illness", an overview, The American Journal of Psychiatry 1994;151,11:1571-1583.
Friedland IR, Jafari H, Ehrett S, Rinderknecht S, Paris M, Coulthard M, Saxen H, Olsen K, and McCracken GH, Comparison of endotoxin release by different antimicrobial agents and the effect on inflammation in experimental Escherichia coli meningitis. J. Infect. Dis. 168:657-662, 1993.
Frieling JTM, Mulder JA, Hendrics T , Curfs JHAJ, van der Linden CJ, Sauerwein RW, Differential Induction of Pro- and Anti-Inflammatory Cytokines in Whole Blood by Bacteria: Effects of Antibiotic Treatment, Antimicrobial Agents and Chemotherapy 35:1439Ð1443, 1997.
Frieling JTM, Van Deuren M, Wijdenes J, Van der Meer JWM, Clement C, Van der Linden C, and Sauerwein RW, Circulating interleukin-6 receptor in patients with sepsis syndrome. J. Infect. Dis. 171:469Ð472, 1995. (see also Frieling et al 1997)
Giambartolomei GH, Dennis VA, Philipp MT, Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys, Infect Immun Jun;66(6):2691-7, 1998.
Goodman and Gilman's "The Pharmacological Basis of Therapeutics, 9th ed., McGraw Hill, 1995, abstracting this value from Schaad et al., 1990.
Groer M, Carr J, Younger MS, Relationships between self-reported symptoms of infection, menstrual-cycle-related distress, and cycle phase. Behav Med 1993 Spring;19(1):13-9, 1993.
Hemmer B, et al. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nature Medicine 5(12):1375-82, 1999.
Hu LT, Klempner MS, Host-pathogen interactions in the immunopathogenesis of Lyme disease. J Clin Immunol 17(5):354-65, 1997.
Hurley JC, Antibiotic-induced release of endotoxin: a reappraisal. Clinical Infectious Diseases 1992;15:840-854.
Karlsson M, Hammers S, Nilsson-Ehle I, Malmborg AS, Wretlind B, Concentrations of doxycycline and penicillin G in sera and cerebrospinal fluid of patients treated for neuroborreliosis. Antimicrob Agents Chemother 1996 May;40(5):1104-7.
Kessler RE, Fung-Tomc J, Susceptibility of bacterial isolates to beta-lactam antibiotics from U.S. clinical trials over a 5-year period, Am J Med 1996;100(suppl 6A):13S-19S.
Klempner MS and Huber BT, Is it thee or me?-autoimmunity in Lyme disease. Nature Medicine 5(12):1346-7, 1999.
Kossmann T, Hans V, Stocker R, Imhof HG, Joos B, Trentz O, Morganti-Kossmann MC, Penetration of cefuroxime into the cerebrospinal fluid of patients with traumatic brain injury. J Antimicrob Chemother 37:161-7, 1996.
Kuby J, Immunology, 3rd Ed., WH Freeman and Co, New York, 1997.
Lawrence C, Lipton RB, Lowy FD, Coyle PK, Seronegative chronic relapsing neuroborreliosis. Eur Neurol 1995;35(2):113-117.
Leslie CA, Dubey DP, Increased PGE2 from human monocytes isolated in the luteal phase of the menstrual cycle. Implications for immunity?, Prostaglandins 1994 Jan;47(1):41-54.
Ma Y, Weis JJ, Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun 1993 Sep;61(9):3843-53.
Mattie H, Antibiotic efficacy in vivo predicted by in vitro activity, Internat J Antimicrob Agents 14:91-98, 2000.
Mattman, LH, Cell Wall Deficient Forms - Stealth Pathogens, 2nd. ed., CRC Press, 1993.
McConnell, J. S., and J. Cohen, Release of endotoxin from Escherichia coli by quinolones. J. Antimicrob. Chemother 18:765-766, 1986. (Letter).
McKenzie FE, Bossert WH, The dynamics of Plasmodium falciparum blood-stage infection, J Theor Biol. 188(1):127-40, 1997.
Mertsola, J., O. Ramilo, M. M. Mustafa, X. Saez-LLorens, E. J. Hansen, and G. H. McCracken, Release of endotoxin after antibiotic treatment of gram-negative bacterial meningitis. Pediatr. Infect. Dis. J. 8:904-906, 1989.
Mohler, J. B. Fantin, J. L. Mainardi, and C. Carbon, Influence of antimicrobial therapy on kinetics of tumor necrosis factor levels in experimental endocarditis caused by Klebsiella pneumoniae. Antimicrob. Agents Chemother. 38:1017-1022, 1994.
Muraille E, Thieffry D, Leo O, Kaufman M, Toxicity and neuroendocrine regulation of the immune response: a model analysis, J Theor Biol. 183(3):285-305, 1996.
Nau R et al., Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother 37:1518-1524, 1993.
Northern AL, Rutter SM, Peterson CM, Cyclic changes in the concentrations of peripheral blood immune cells during the normal menstrual cycle. Proc Soc Exp Biol Med Oct;207(1):81-8, 1994.
Phillips SE, Mattman LH, Hulinska D, Moayad H, A Proposal for the reliable culture of Borrelia burgdorferi from patients with chronic Lyme disease, even from those previously aggressively treated, Infection 26 (1998) 364-367.
Preac-Mursic V, Wilske B, Schierz G, Holmburger M, Süß E, In vitro and in vivo susceptibility of Borrelia burgdorferi, Europ J Clin Microbiol 6:424-426, 1987.
Preac-Mursic V, Weber K, Pfister HW, Wilske B, Gross B, Baumann A, Prokop J, Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection Nov-Dec;17(6):355-9, 1989.
Preac-Mursic V, Wilske B, Schierz G, Süß E, Comparative antimicrobial activity of new macrolides against Borrelia burgdorferi. Europ J clin Microbiol Infect Dis 8:651-653, 1989.
Preac-Mursic V, Marget W, Busch U, Pleterski D, Rigler S, Hagl S , Kill Kinetics of Borrelia burgdorferi, Infection, 24: 9 - 16, 1996.
Preac Mursic V, Wanner G, Reinhardt S, Busch U, Marget W, Formation and cultivation of Borrelia burgdorferi spheroplast-L-form variants. Infection 24 (1996): 218-226.
Roumen RMH., Frieling JT M , van Tits HWHJ , van der Vliet JA, and Goris RJA, Endotoxemia following major vascular operations. J. Vasc. Surg. 18:853-857, 1993.
Samples taken from 43 year old female whose data were analyzed in this report.
Schaad UB, Suter S, Gianella-Borradori A, Pfenninger J, Auckenthaler R, Bernath O, Chesaux JJ, and Wedgwood J, A comparison of ceftriaxone and cefuroxime for the treatment of bacterial meningitis in children. N. Engl. J. Med. 322:141-147, 1990.
Sellati TJ, Abrescia LD, Radolf JD, Furie MB. Outer surface lipoproteins of Borrelia burgdorferi activate vascular endothelium in vitro. Infect Immun Aug;64(8):3180-7, 1996.
Schutzer SE, Coyle PK, Belman AL, Golightly MG, Drulle J, Sequestration of antibody to Borrelia burgdorferi in immune complexes in seronegative Lyme disease. Lancet 1990 Feb 10;335(8685):312-315.
Schutzer SE, Coyle PK, Dunn JJ, Luft BJ, Brunner M, Early and specific antibody response to OspA in Lyme Disease. J Clin Invest Jul;1994(1):454-457, 1994.
Shenep, JL, Flynn PM , Barrett FF, Stidham GL, and Westenkirchner DF, Serial quantitation of endotoxemia and bacteremia during therapy for gram-negative bacterial sepsis. J. Infect. Dis. 157:565-568, 1988.
Shenep, JL, Barton RP, and Mogan KA, Role of antibiotic class in the rate of liberation of endotoxin during therapy for experimental gram-negative bacterial sepsis. J. Infect. Dis. 151:1012-1018, 1985.
Shmuklarsky MJ, Boudreau EF, Pang LW, Smith JI, Schneider I, Fleckenstein L, Abdelrahim MM, Canfield CJ, Schuster B, Failure of doxycycline as a causal prophylactic agent against Plasmodium falciparum malaria in healthy nonimmune volunteers. Ann Intern Med 1994 Feb 15;120(4):294-9.
Sigal LH, Immunologic mechanisms in Lyme neuroborreliosis: the potential role of autoimmunity and molecular mimicry, Semin Neurol 1997 Mar;17(1):63-8
Sigal LH, Williams S, A monoclonal antibody to Borrelia burgdorferi flagellin modifies neuroblastoma cell neuritogenesis in vitro: a possible role for autoimmunity in the neuropathy of Lyme disease, Infect Immun 1997 May;65(5):1722-8.
Smirnova OA, Study of cyclic kinetics of immunity by mathematical modeling methods, Kosm Biol Aviakosm Med. 25(5):53-6, 1991.
Spangler SK, Jacobs MR, Apfelbaum PC, MIC and Time-Kill Studies of Antipneumococcal Activity of 118819X (Sanfetrinem) Compared with Those of Other Agents, Antimicrob Agents Chemother 41:141-155, 1997.
Straubinger RK, Straubinger AF, Summers BA, Erb HN, Härter L, Appel MJG, Borrelia burgdorferi induces the production and release of proinflammatory cytokines in canine synovial explant cultures. Infect Immun 66(1):246-258, 1998.
Straubinger RK, PCR-Based Quantification of Borrelia burgdorferi Organisms in Canine Tissues over a 500-Day Postinfection Period. J Clin Microbiol 2000;38:2191.
Tai KF, Ma Y, Weis JJ, 1994. Normal human B lymphocytes and mononuclear cells respond to the mitogenic and cytokine-stimulatory activities of Borrelia burgdorferi and its lipoprotein OspA. Infect Immun Feb;62(2):520-8, 1994.
Thornsberry et al., In vitro activity of cefepime and other antimicrobials: survey of European isolates, J. Antimicrobial Chemother 32, Suppl. B, 55-62, 1993.
van den Berg C, De Neeling AJ, Schot CS, Hustinx WMN, Wemer J, and de Wildt DJ, Delayed antibiotic-induced lysis of Escherichia coli in vitro is correlated with enhancement of LPS release. Scand. J. Infect. Dis. 24:619-627, 1992.
van Deuren, M, van der Ven-Jongekrijg J, Bartelink AK, van Dalen R, Sauerwein RW, and van der Meer JW, Correlation between proin-flammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J. Infect. Dis. 172:433Ð439, 1995.
Wagner D private communication, Pharmaceutical Info Div., 777 Scudders Mill Road, Plainsboro, NJ 08536, Tel.: +1 (609) 897 - 2776, Fax: +1 (609) 897 - 6042.
Yim CW, Flynn NM, Fitzgerald FT, Penetration of oral doxycycline into the cerebrospinal fluid of patients with latent or neurosyphilis.Antimicrob Agents Chemother 1985 Aug;28(2):347-8.
Zhang JR, Hardham JM, Barbour AG, Norris SJ, Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes, Cell Apr 18;89(2):275-285, 1997.
Zhang H, Niesel DW, Peterson JW, Gary R, Lipoprotein release by bacteria: potential factor in bacterial pathogenesis, Infect Immun 66(11):5196-5201, 1998.
Bb = Borrelia burgdorferi.
C(t) = concentration of substance provoking immune response, i.e. of Bb (CBb(t)) and of Bb fragments (CF(t)) (units: number of spirochetes per system volume).
cCSF(t) = concentration of antibiotic in CSF (units: mg/L).
cGI(t) = concentration of antibiotic in gastro-intestinal tract at time t after drug intake (units: mg/L).
CHx = threshold concentration of Bb fragments starting Herxheimer like reaction (units: number of fragments per system volume).
cP(t) = concentration of antibiotic in blood plasma at time t after drug intake/infusion (units: mg/L).
CooBb = stationary concentration of Bb population outside the niche = r(t)/(ln2 (1/TBb + 1/TIBb)) or r(t) TBb/ln2, depending on whether immune system is assumed to be eliminting Bb or not (units: number of fragments per system volume).
CooF(t) = concentration of Bb fragments after concentration CBb(t) of Bb population outside the niche has reached its stationary value CooBb (see Fig. 12) (units: number of fragments per system volume).
compartment model = a visualization of a linear system of first order differential equations describing the growth of the number of cells in a system. A system may consist of several subsystems, each of which will be represented by a compartment. Compartments have in- ond outfluxes having the dimension cells per time (when the entities within a compartment are cells). What comes out of one compartment may go into some other compatrment, the two compartments being "coupled". Each compartment is represented by a differential equation which states how much goes in and out per unit time. The Mathematica code representing the compartment models used here is given in http://www.lymenet.de/symptoms/cycles/mathcode.htm.
cytokines: Plasma LPS concentrations usually do not correlate with clinical symptoms (Roumen et al. 1993). It is the induction of cytokines through cell wall components like LPS which mediates the biological responses during bacterial infections. Cytokine levels and types of cytokines have repeatedly been shown to correlate with clinical outcome (Damas et al., 1992, Frieling et al., 1995, van Deuren et al., 1995).
C1 = threshold concentration triggering the immune system to start toxin elimination (apparently by its humoral branch). The immune response starts with a lag phase. Immune response subsides when toxin concentrations "visible" to the immune system have fallen below another threshold concentration. (units: number of spirochetes or fragments per system volume).
C2 = concentration threshold for inflammation, i.e. above which illness symptoms are perceived (units: number of spirochetes or fragments per system volume).
D = dosage of cefuroxime (gram per intake).
deltai = length of the ith menstrual cycle.
Delta t = time during which cefuroxime concentration in CSF is larger or equal a given inhibitory concentration (units: hours/day).
Delta tsubinh = time during which cefuroxime concentration in CSF is smaller than a given inhibitory concentration (units: hours/day).
equilibrium of a compartment = state in which influx to the compartment equals outflux out of it. At equilibrium the compartment is full, meaning that its concentration will no longer rise.
In particular, here are some properties of the 2-compartment system in Fig. 12:
(1)
CBb(t)' = r(t) - ln2 CBb(t) (1/TBb + fBb/TIBb)
(2)
CF(t)' = ln2 (CBb(t)/TBb - fF CF(t)/TF)
(5)
r = ln2 CBbeq (1/TBb + fBb/TIBb)
These are the values between which the dashed curve in Fig. 12 oscillates.
F = subscript meaning Bb fragments.
f = free (i.e. Bb affecting) fraction of cefuroxime concentration in considered subsystem (here CSF) relative to its plasma concentration (f = : 1 for plasma), f = cCSF/cplasma = 0.1 for CSF (dimensionless). Data from
f(C, t) = dimensionless function describing the activity of the immune response ("immune switching function"). f ia either 0 ("no immune response") or 1 ("immune response"). Here, the immune response is assumed to be directed
flare = cluster of days with symptom occurrence.
follicular phase (here used sensu lato) = the first phase of the menstrual cycle, starting with the menstruation (menstrual bleeding) and ending with the ovulation, i.e. days 1 through 12 ... 14.
I = superscript meaning immune system.
incubation time = time between infection (entrance of the pathogen into host) and development of clinical symptoms.
Immune Response Interval = time interval of approximately 6 days duration, centered around the day of menses (beginning of menstrual bleeding), during which Barkley, Harris and Szantyr observed systematically high antigen concentrations in the urine of a Lyme patient (Barkley et al., 1997). The authors suggest that the immune system has a higher level of activity during this phase (see also testimony of M.S. Barkley before the New York State Assembly Standing Committee on Health, Public Hearing "Chronic Lyme Disease and Long-Term Antibiotic Treatment", Albany, NY, USA, 27.11.2001, pp. 199 - 227).
invisible = located in a compartment into which the immune system or the antibiotic penetrates only poorly. The table gives examples of such locations in which Bb were found.
k
i =
kPCSF = transition rate for drug from plasma to CSF compartment.
lag time = lag phase (symbol: tau)
ln 2 = (natural logarithm of 2 =) 0.69.
LPS = lipopolysaccharide.
luteal phase = phase of the menstrual cycle after the ovulation.
memory = the attribute of the immune system mediated by memory cells whereby a second encounter with an antigen induces a faster start and a heightened state of immune reactivity (Kuby pp 397 - 399)
menses = day of onset of menstrual bleeding.
MIC = Minimum Inhibitory Concentration. Definition: MIC is the minimum level necessary to inhibit bacterial growth. It depends on the bacterial isolate. MIC50 and MIC90 are the levels at which 50 % and 90 % of the tested isolates are inhibited, respectively.
ni = number of days of a constant cefuroxime regimen (units: day).
net growth of Bb population = growth of population remaining when decay of population has been subtracted.
niches protect Bb from the immune system or the antibiotic (Preac-Mursic et al., 1989) or render toxin released by Bb "invisible" to the immune system. The protection may wane with time and so will the size of the spirochete or toxin population in the niche.
Niches are provided by the host in the form of physical compartments, but they can also be produced by Bb itself in the form of chemical or microbiological defense mechanisms (see also overview in Chapter Background Information of J.J. Burrascano's essay "Managing Lyme Disease".
The basic concept underlying the model is that the niche has the following properties:
The literature gives examples of such locations (i.e. intracellular locations) in which Bb were found. See also literature on mechanisms of cell invasion.
Osp = variable, plasmid encoded Outer sphere protein of Bb. The Osp's labeled OspA (30 ... 32 kD), OspB (34 ... 36 kD), OspC (21 ... 24 kD) are unique for Bb, as are the proteins p39 (39 kD) and p93 (93 kD).
ovulation = day on which the ripe ovum (egg cell) leaves the ovarian follicle.
prostaglandine E2 = a lipid inflammatory mediator with diverse biological activities, including increased vascular permeability and dilation, and induction of neutrophil chemotaxis (Kuby, p. 368-371).
r(t) = time variable Bb source term in compartment model (units: spirochetes per day entering unit system volume). It is assumed that r(t) varies much more slowly than concentrations C(t).
symptom = consequence of an inflammation of glial or neural tissue.
system = infected organ or tissue responsible for symptom. Basic systems are defined after Bleiweiss and have been further expanded here into subsystems characterized by the symptoms in Fig. 1.
ta = part of flare cycle during which immune system is active (units: day).
tb = part of flare cycle during which immune system is not yet active (flare cycle duration is tb + ta) (units: day).
TBb = in vivo Bb generation time (units: day).
TBbinvitro = in vitro Bb generation time. Values extracted tentatively from kill kinetics published by Agger et al. and Preac-Mursic et al. are 11 hours and 10 hours, respectively.
TBbI = Bb elimination half life characterizing immune system (units: day).
TCSF = elimination half life of antibiotic from CSF compartment (units: hour).
TFI = Bb fragment elimination half life characterizing immune system (units: day).
TGI = elimination half life for GI-tract resorption.
total concentration of drug = concentration of all chemical species of drug. Chemical species are the free drug and chemical complexes containing drug. Total concentrations are determined by breaking up all chemical complexes. (units: mg/l).
Via molecular mimicry (Kuby, Ch. 20, S. 497), also autoimmune processes can be triggered by Bb proteins (Sigal 1997, Sigal and Williams 1997). T-cell subpopulations (of short-lived T-cells) responsible for autoimmune processes might persist as long as a sufficient level of such proteins exists.
TP = renal elimination half life from plasma compartment.
t = time variable (units: hour in pharmacokinetic model, units: day in models for flare cycles, Figs. 11 and 12).
tinet = time for net growth of Bb population.
t0 = time of bolus infusion of cephalosporin (units: hour).
tau = lag time for resorption from GI tract. tau = 1.4 h, fitted from experimentally determined plasma concentrations (units: hour).
22.1 mg/l = peak plasma concentration measured in patient's plasma after intake of 2 gram of cefuroxime with prior meal.
xyz =: n this equation means xyz is by definition equal to n.
= (evolution) time between immune system alert by antigen and beginning of antigen elimination during which naive B cells undergo clonal selection in response to the antigen. Lag in primary immune response: generally 4 ... 7 days (as opposed to the lag phase in secondary, i.e. memory enhanced, immune response, which ranges generally between 1 and 3 days). Time of peak response: primary response: 7 ... 10 days, secondary response: 3 ... 5 days (Kuby, pp 397 - 399). Inflammation caused be Osp is not modulated by immune memory.
= time between beginning of infusion and appearance of infused drug in CSF. Equation for ccsf in box in Fig. VIII. 5.5 assumes lag time = 0.
MIC for cefuroxime = 0.13 mg/L, as determined by Agger et al. 2 MIC = 0.26 mg/L is used in the computations, which corresponds to MIC90 = 0.25 mg/L as determined by Preac-Mursic (1987). A critical discussion of the concept behind the MIC can be found in Mattie H 2000) See also Preac-Mursic et al 1996.
In the model simulating the flare cycles in the presence of antibiotics, the term "niche" is used in this generalized sense.
These toxins produce cytokines (Ma et al. 1993, Tai et al. 1994, Sellati et al. 1996, Frieling et al. 1997, Burns et al. 1998, Giambartolomei et al. 1998, Straubinger et al. 1998, Zhang et al. 1998, see also the result of a Medline search). It is the cytokine levels that correlate with clinical responses (Damas et al., 1992, Frieling et al., 1995, van Deuren et al., 1995).
(see also lag phase in immune response.)
(*) This chapter is part of a draft: "Lyme Disease: Statistical Evaluation of a
Symptom Log and an
Empirical Theory of Flare Cycles